1
Australia has an extraordinary diversity of birds and a fascinating paleontological history (Schodde 2005a). Because the two may belong inextricably together, they need more than a fleeting mention. Climate and land contribute immeasurably to the way in which an organism can develop, diversify and radiate. Climate, for instance, may force migration or encourage permanent settlement. The land may provide for all needs or only for some of them, or it may be unreliable and fickle in the way in which it can support life and, in this case, animals that stay need to have their wits about them. If climate permits and birds can defend year-round all-purpose territories, then this may have social consequences as well: breeding seasons can lengthen and partners may not need to separate after each breeding season. Unreliable food sources or involuntary exposure to many novel situations exerts pressure which in turn may lead to the evolution of a larger brain because this, as some have argued, may require increased behavioural flexibility and lead to the ability to cope with and respond to novel challenges. Indeed, certain circumstances within climate and landscape may require sophisticated perceptual and cognitively advanced abilities (Amiel et al. 2011). Conversely, if chances of survival are better with a bigger brain and greater cognitive abilities, then time and energy are needed to nurture and support a bigger brain (see Chapter 2) and conditions in the environment need to make this possible.
Hence the question where birds first evolved is crucial. This had been a puzzle for a long time and is not a marginal question or of limited interest only to Australia. To trace species to their origin can, after all, also trace certain traits and expose mechanisms of evolution. It is very pleasing to be able to say at the beginning of this book that the question of the origin of birds appears to have been settled once and for all. In 2004, a very telling American paper was published about the origin of birds (Barker et al. 2004). It made headlines everywhere and had an even more important response within Australia. It showed conclusively that passerines (perching birds comprising true songbirds called ‘oscines’ and sub-oscines, that make up two-thirds of the world’s existing avian species) originated in the parts of Gondwana that comprise present-day Australia, New Zealand and Antarctica (Cracraft 2001; Barker et al. 2004). This was a landmark publication and laid to rest a 200-year controversy between southern and northern hemisphere scholars.
The Australian Museum and researchers, such as Christidis and many other scholars in Australia, had argued for decades that the origin of birds was Gondwana. When interviewed for his response to this publication by the ABC Online science program (Skatssoon 2004), Christidis said that the suggestion that songbirds originated in Australia had been considered ‘ludicrous’ earlier when presented by Australian researchers because, as most believed, Australia did not have that many birds relative to the rest of the world and so it could hardly be central to avian evolution. When Christides and colleagues first presented their results in the 1980s, they were ‘laughed at’ by their northern hemisphere colleagues. ‘Up until the last four or five years it's always been thought that the passerine birds originated in the northern hemisphere and spread south and that's been the gospel for the last 200 years,’ Christides was quoted as saying (Skatssoon 2004).
There was another major finding in 1997, showing conclusively that the worldwide mass-extinction event of 65 million years ago that had wiped out dinosaurs completely, did not end bird life. Indeed, Cooper and Penny (1997) found evidence for a mass survival of birds of at least 22 lineages, suggesting diversification already during the Cretaceous period 141–65 million years ago (Cooper and Penny 1997). Hence, according to this latest understanding, modern lineages did not arise after the mass extinction event but well before it, and knowledge of this then made it imperative to know when and in what sequence the various continents and land masses separated and which living cargo they took with them and which species evolved locally only after the supercontinent split apart.
Because of better technologies and new methods for measurements in the last 15–20 years, all these fields of research just about co-timed their new discoveries in such a manner that they could inform each other, leading to a virtual explosion of publications and new findings in the last 10 years. The very first and significant fossil find in Australia was the discovery by Boles of a songbird fossil carbon-dated as being about 54 million years old (Boles 1995). It is the oldest songbird fossil ever found. The next earliest to it is a recent find in Europe, dated to about 30 million years ago (Mayr and Manegold 2004). Clarke et al. (2005) reported a fossil find in Antarctica that represented the first Cretaceous fossil definitively placed within the extant bird radiation. It was a new species named Vegavis iaai and identified as belonging to Anseriformes (waterfowl) and closely related to Anatidae, which include true ducks. They inferred and concluded that at least relatives of ducks, chickens and ratite birds were living at the same time as non-avian dinosaurs (Clarke et al. 2005). Knowing now that waterfowl and ratites, of which the emu is a descendant (Fig. 1.1), belonged to very old lineages derived from the Australian part of Gondwanaland before separation from Antarctica allows us to trace the evolution of lineages not only along genetic and species lines but also in terms of the evolution of behaviour (to be dealt with in the next chapters).
In 2013, a fossil find of avian footprints discovered among the fossil-rich cliffs of Dinosaur Cove on the coast of southern Victoria caused another media stir (Sci-News.com 2013; Gannon 2013). The researchers think the tracks belonged to a species about the size of a great egret or a small heron (Martin et al. 2014). The find was identified as being about 105 million years old, placing it in the Early Cretaceous period and making this the oldest avian footprints ever found in the world (Fig. 1.2). This places the evolution of birds not merely well within the Cretaceous period but even towards the very beginning of this period.
A further landmark for avian evolution came from genome sequencing. By the end of 2013, the genomes of about 50 mammalian species had been sequenced while those of just seven bird species had been determined. The first was the chicken (Hillier et al. 2004), the second the zebra finch (Warren et al. 2010), followed by the turkey (Dalloul et al. 2010) and, among others, also the peregrine and saker falcons (Wang et al. 2013). In 2014, the budgerigar sequencing was published (Ganapathy et al. 2014) and some impressive genomics comparisons in birds were undertaken covering sequencing of a further 41 bird species allowing new and elucidating comments relevant to taxonomy (Zhang et al. 2014; Jarvis et al. 2014) and brain evolution, specifically with evolution of song and language in mind (Pfennig et al. 2014). No doubt, as a result of these substantial and new analyses, the next decade will bring further insights and new perspectives on avian evolution, diversification and radiation and a whole host of new information. They may even help elucidate how and when certain brain structures appeared in songbirds or even in specific species, such as the cockatoos, that have particularly large brains. We now have the means to probe the links between genes, brain, behaviour, environment and evolution. Indeed, such research has already started (Clayton et al. 2009).

Fig. 1.1. The emu derives from one of the oldest lineages of birds. It is particularly significant as one of the animals on the official crest of Australia. Emus (like other ratites) are unusual in that the male collects and broods the eggs of several females and protects the hatchlings until they can fend for themselves. Indeed, it is a complete role reversal. Females also initiate courtship.

Fig. 1.2. The oldest footprints of a bird (105 million years old) with the feet of an extant species superimposed. Very little change in the design of feet is apparent. The researchers regard the smudges on the left foot as an indication that the bird was just soft-landing from flight. This tantalising thought hints at the possibility that these early modern birds were perhaps not just gliding but had already acquired flight. The left footprint is not unlike that of a lyrebird. (Adapted from lifescience.com 25 Oct. 2013, and sci-news.com and Emory Health Sciences 28 Oct. 2013 online, www.sciencedaily.com)
At least the debate about the local origin of songbirds and many other orders seems to have been laid to rest, but it is worth remembering that this occurred as recently as 2004 and there are many unanswered taxonomical questions yet to resolve. We may well find, also in view of the results of comparative genomics, that some Australian avian species may still be reassigned to a different family or may become their own family in the near future (Christidis and Norman 2010). Investigations into the evolutionary history of birds are aided by better techniques, spectacular new findings and by more data points over many years of a lively taxonomy and paleontological culture in Australia. To this day, native Australian birds are being reassigned and renamed taxonomically (Brown et al. 2008). Moreover, species are still being discovered, mostly at the recent and extremely productive fossil site of Riversleigh in north-western Queensland such as a new parrot, stork, swiftlet and gallinule from the Tertiary to the Miocene (Boles 1993, 2001, 2005a, b, c).
So far confirmed is that, among non-songbirds, ratites, chicken, ducks, pigeons and parrots evolved in that part of Gondwana that includes present-day Australia and New Zealand. And then there are the perching birds, of which there are nearly 5800 species now, constituting 60% of all birds worldwide. Previous genetic studies had already supported the hypothesis that honeyeaters are an ancient radiation within Australasia (Sibley and Ahlquist 1985, 1990; Christidis and Schodde 1991). The earliest lineages to which lyrebirds and scrub-birds, treecreepers, bowerbirds and catbirds belong are thought to have evolved 53–45 million years ago. The next were the bristlebirds, pardalotes, fairy-wrens and scrubwrens about 35 million years ago. As far as we know, thereafter followed Corvida and Passerida. Core Corvoidea include the Australasian centred fantails, birds of paradise, whistlers, magpies, butcherbirds, currawongs, woodswallows, flycatchers, cuckoo-shrikes, orioles and crows. They seem to have evolved about 20 million years ago. By that time, the scleromorphous woodlands were already well established in Australia and increasing aridity and warming, and possibly also deficient soil conditions, low in calcium and nitrogen, changed the flora and encouraged the diversification of scleromorphous woodlands (Groves 1994). It has been argued that the spread of open woodlands and grasslands fostered the existence and spread of Australia’s giant herbivorous ground birds, such as the dromornithids, also called Mihirungs (Murray and Vickers-Rich 2004). Paleoclimate details of the last 250 000 or so years suggest some complex patterns of glacial and interglacial climate shifts with weakening northern monsoon rains. Rainforests receded; the land dried further and vast grasslands spread. The changes harmed some species, specifically the megafauna that became largely extinct, including the Mihirungs (Murray and Vickers-Rich 2004), but it benefited others. For instance, the spread of the family Artamidae and Cracticus (magpies and butcherbirds and allies) has been seen as tied to climatic changes and vegetation type (Kearns et al. 2011, 2013). This information gleaned from the paleontological literature (Christidis and Norman 2010) is convincing evidence enough for me to consider Australia and birds as belonging together and as inextricably intertwined. It is a question what the role of climate changes, innovation and cooperation might have had in them surviving and thriving.
Shape of Australia after break-off from Gondwana and bird life
The date of Australia’s final breaking off from the remainder of Gondwana and the precise shape of Australia as a landmass at the time of its breakaway seem to be relatively uncertain. Around 50 million years ago, well after the mass extinction, the shape of Australia is depicted as something very different in various publications. The Australian continent was nothing like the shape it is today and, to my surprise, I found little agreement as to which part of the continent was, at any one time in its paleogeographic past, really part of the landmass and which was submerged or formed islands. It is very clear, however, that in the Cretaceous period (i.e. in the millions of years when modern birds first evolved) mainland Australia was not one island and not a unified landmass (although it had the potential to be so depending on sea levels), but a myriad of islands because of rising sea levels (Fig. 1.3). Sources seem to agree that rising sea levels caused the large flat part of inland Australia to flood, allowing the sea to invade or even divide eastern and western Australia for probably tens of millions of years. These periods happen to roughly coincide with the now suspected period of evolution of modern bird lineages. Even at times of dominant landmasses, there were two large landmasses that were separated by substantial bodies of water, oceans that were unbroken by land from the north right down to Antarctica at the very time when birds are thought to have evolved (although it is not clear whether some parts of the southern end of Australia were submerged or not). Some publications for the general reader draw maps that are completely land while others draw contours that suggest that the continent was partly split into east and west. The myth of an inland sea may have arisen partly out of the knowledge that sea creatures were found in what is now central Australia and can be seen displayed in Coober Pedy. It is hard to imagine that the land on which Coober Pedy was built – now the opal-mining centre of Australia and very much part of dry and hot inland Australia – was part of the ocean floor 120 million years ago and beyond. If part of the continent had consisted of many islands at the time when modern birds first evolved, this might well be of major significance. We know that oceanic islands drive divergence and speciation, but also have a potential role as repositories of ancestral diversity (Aleixandre et al. 2013).

Fig. 1.3. Australia 110 million years ago (thick black outline). The continent is far from being one landmass – with present-day Australia shown in white. This ‘inland’ sea is called the Eromanga Sea. It has yielded rich fossil finds of sea mammals. (Redrawn and simplified from Laseron 1984.)
Australia, at least for possibly a few crucial tens of millions of years, might have been a thousand islands, not one landmass in which species were readily able to criss-cross the continent. Many islands foster speciation and are said to have had an influence on brain evolution as well (Amiel et al. 2011). And much later, when Australia became one landmass (due to substantial falls in sea levels) and took on a shape similar to that of today, it then sported an appendage that was part of its tectonic plate: namely a land bridge to Papua New Guinea that spanned hundreds of kilometres and created an easy passage for birds to get to South-East Asia and for Papuan species to cross over to Australia. The extraordinary outcome is that the Australian landmass now has different ages in terms of emergence from the water – some areas in Queensland and in Western Australia have always been land but many others have a more recent history.
One of the starkest reminders of the ancient, but variously exposed, parts of the Australian continent are the unique sites in South Australia revealing Precambrian soft-bodied Ediacara Biota, dating back over 500 million years and showing so far the very first evidence worldwide of the emergence of mobile life forms (Gehling and Droser 2012). The fossils found in South Australia were once part of the sea floor. They now give us the first glimpse of organisms that became the template for all viable life forms from invertebrates to humans (Peterson et al. 2008). There can be nothing more ancient in a continent than such finds and these are on Australian soil and part of the patchwork of the current continental shape and composition that may have changed every so often and in significant ways: from land back to sea and back to land. This, as much or more so than even climate change, might have helped shape Australia’s bird life, and all of its other flora and fauna.
In summary, the current shape of Australia is not what it was at the time birds evolved due to: (a) changing sea levels separating parts of Australia from one another; (b) an important land bridge with Papua New Guinea, at least for some time; and (c) a receding coastline. Modern Australia, as a continent, is a patchwork, a quilt of parts that became exposed to become land at various times in prehistory and these phenomena present challenges when attempting to determine the biogeographical patterns of dispersal and diversification in birds (Jønsson and Fjeldså 2006; Schodde 2005b; Kearns et al. 2011).
It is very exciting to think that many passerine birds found along the east coast of Australia, such as lyrebirds, bowerbirds, treecreepers and honeyeaters, are living descendants of tens of millions of years of evolution, and they are not the only ones.
There are places in Australia where one knows one walks on millions of years of natural history, such as the extraordinary fossil sites of Riversleigh, Winton and Dinosaur Cove in southern Victoria, mentioned above, and other important sites that have yielded discoveries of remnants of bird bones. In addition, there are extant birds in special areas in the current shape of the Australian landmass that are both informative and revealing about the very special conditions of the Australian continent.
One of these special places, researched in detail by teams from Australian National University, is the Cape York Peninsula. Heinsohn and Cermak wrote that it had its own ornithological story to tell about the evolutionary ecology of birds of national and international importance. They wrote in their introduction:
The wildlife of Cape York Peninsula comprises a fascinating mix of ancient Australian species together with many recent arrivals from New Guinea and South-East Asia. This is because the tip of Cape York is only separated from New Guinea by a narrow and shallow channel of water, Torres Strait, and in the not-so-distant past the two landmasses have been joined by massive land bridges whenever sea levels have been lower. These bridges extended the area of land considerably and provided vast tracts of habitat, including entire river systems, lakes, swamps, woodlands, heaths and forests linking the wildlife of the two regions. The birds of CYP have an important story to tell because of their diverse evolutionary origins, and because they vary greatly in their mobility from sedentary species to long distance migrants. This makes their present day distribution and movements unusually informative for understanding our evolutionary past, including our original Gondwanan heritage, further evolution in isolation, and eventual re-connection with the rest of the world (Heinsohn and Cermak 2008).
The world has had good reason to think about climate change and changing sea levels. None is more acute than in Australia, already the driest continent and one that has been affected by changing sea levels in one way or another over millions of years. There is no writer on the evolution of bird life in Australia who is not keenly aware of the role of changes of sea-levels, climate and the geology itself as major driving forces in the diversification (meaning both speciation and extinction) of birds in the Australo-Papuan region (Kearns et al. 2013). Research in some environments, especially in the sea and the Great Barrier Reef and in specific bird hotspots, has been ongoing for many years and has had the input of many very dedicated, progressively thinking and enlightened people and researchers.
The main question here is not only what kind of challenges Australian birds may face in the future but what challenges they, as species, have already survived and why or, at least, what strategies may have some explanatory power about their survival and their specific life histories and behaviour. These may include questions about longevity, cooperative breeding and their relationship to a general, and even specific, intelligence – variables that are increasingly seen as compelling for explaining features of the biology and behaviour of birds.
Australia a hotspot for cooperative behaviour in birds
A topic that is largely specific to Australian avifauna concerns the banding together of birds into groups and cooperative units. The levels of cooperation are often unparalleled, as will be discussed throughout this book. Instances of cooperative behaviour in birds can be found worldwide, although in a highly uneven distribution. The largest clusters are in Australia and in the tropics so it is not surprising that its discovery was first made in Australia (Boland and Cockburn 2002). For vast stretches of Eurasia and the Americas, species showing cooperative behaviour rank below 2%, or in some pockets up to 5%, but in Australia cooperative behaviour is known in up to a quarter of all avian species (see the excellent and useful maps of the worldwide distribution of cooperative behaviour in Feeney et al. 2013). There must have been good evolutionary reasons for this to have occurred, but it is not clear whether cooperation is an ancient type of social behaviour or whether it evolved later. It has also been asked whether cooperation at group level may be the ancestral form while pair bonding may have been a later innovation (Nicholls et al. 2000).
Andrew Cockburn of the Australian National University has done substantially more than indicating cooperative behaviour. He completed a monumental task by publishing one of the most complete lists of parenting of offspring in birds ever assembled (Cockburn 2006). He checked on all 9456 avian species in the world and was able to find sufficient data for 54% of them, or 5143 species worldwide. He then subdivided these into several subcategories of parental care from no care at all to cooperative group rearing and found that, of the known species, a staggering 81% of birds were raised by both parent birds and a further 9% by more than two (group/helpers, etc.) – the remainder of 10% was made up of various groups including lone parenting (either male or female but usually female), of those incubated by geothermal heat, the megapodes (Fig. 2.2), and a group of birds that parasitise other nests (the last group, incidentally, would add to the bi-parental figures).
The Australian continent has examples of the entire range of reproductive strategies in birds (see Appendix 1). Some species do not raise their offspring at all. No care at all after hatching is the rare and drastic solution taken by megapodes such as malleefowl and brush turkeys. Then there are the cuckoos, 12 species in all, or 13 if one counts the oriental cuckoo as a summer migrant to the Top End. Except for the pheasant coucal that builds a nest and rears its own young, all others are brood parasites and leave the raising of their offspring – one or even two – to another species of bird. Each species has different preferences. The channel-billed cuckoo, the largest cuckoo in the world, prefers currawongs, Australian magpies and ravens as hosts for its offspring (Fig. 1.4). The eggs of the channel-billed cuckoo resemble those of currawongs and magpies and perhaps the magpie pairs continue to be fooled because the palate (i.e. the inside of the beak exposed during begging for food) in a cuckoo hatchling is a bright red colour as is the palate of magpie hatchlings or those of currawong.

Fig. 1.4. The juvenile channel-billed cuckoo (on the left) is already over half a metre in length. It seems to be unknown why the channel-billed cuckoo has evolved to be of such extremely large size – even currawongs and magpies have difficulties providing enough food for this large bird. The bill is so large that the entire head of an adult host bird can fit into it. Here a currawong (on the right) is feeding the fledgling.
Given the number of cuckoo species and the high level of cooperative behaviour, some researchers have wondered whether cuckoos might in fact be ecological drivers of the persistence of cooperative breeding because they are intimately linked to species that breed cooperatively (Feeney et al. 2013).
Most Australian birds pair bond and many do so for life. Cooperative breeding means that, in addition to the stable parental pair, there may be helpers. Cooperative breeding in birds describes social systems wherein more than two individuals combine to rear a single brood of young (Cockburn 1998). In the traditional sense of the definition of cooperation (i.e. ‘helping at the nest’), individuals in addition to the parents, assist in the daily care and feeding of nestlings and in defence of juveniles that are clearly not their own. The helpers are usually siblings and offspring of a previous season but sometimes even this genetic link is not present. The majority of cooperative species may fall into the former category, as do magpies, while in kookaburras the helpers always seem to be offspring from a previous season.
Heinsohn and Double called the Australasian region, with 115 cooperatively breeding species, the world hotspot for cooperatively breeding birds (Heinsohn and Double 2004). Cooperative breeding in birds is thus much more prevalent than had been realised previously, occurring in 18.5% of songbirds known to have biparental care. If only the old Australian avian lineages mentioned above are considered, as Cockburn (2003) pointed out, cooperative breeding even becomes the predominant social system rising to an astounding 51% (79 out of 155 species). Interestingly, most cooperative species seem to cluster in specific clades (Fig. 1.5).
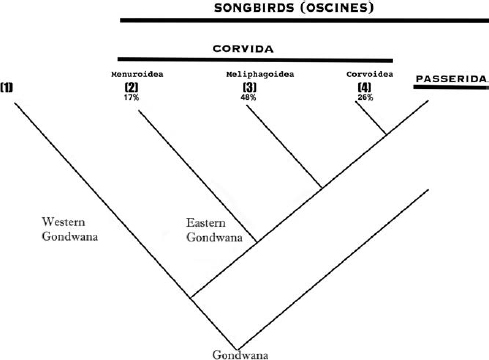
Fig. 1.5. Taxonomy of the groups of landbirds specially discussed in this book. The numbers represent specific clades and the percentages represent the number of cooperative species within each group. (1) The suboscines such as pittas– not fully classed as songbirds; (2–4) represent the large groups of birds for which some overall classification of Corvida was given (some classification that has been under review and partially abolished in recent years, although it has some merits in terms of this book). (2) The Menuroides comprising quite disparate ancient root lineages such as lyrebirds and scrub-birds, treecreepers and bowerbirds. (3) The Meliphagoides comprising the large group of all honeyeaters, fairy-wrens, pardalotes and acanthizid warblers. (4) The Corvoidea comprising a very disparate group of Australasian robins and babblers, whipbirds, magpies, butcherbirds, woodswallows and currawongs, Old World orioles, cuckoo-shrikes, birds-of-paradise, mud nesters (white-winged choughs, apostlebirds), whistlers, fantails, monarchs, sitellas, quail-thrushes, ravens and crows.
Among the most studied and reported species are the superb fairy-wren (Cockburn et al. 2003; Langmore et al. 2012), the white-winged chough (Heinsohn 1991a,b, 1992), apostlebirds (Chapman 1998; Woxvold 2004), grey-crowned babblers (King 1980; Blackmore and Heinsohn 2007), Hall’s babblers (Balda and Brown 1977), sitellas and dusky woodswallows (Davis 2006). Probably the best-known, but not necessarily consistently, cooperative species are the Australian magpies (Kaplan 2005), kookaburras (Eastman 1970) or white-naped honeyeaters (Harrison 1969; Newman et al. 1970).
While this definition of cooperative behaviour sounds simple and easy to grasp, it is actually not so simple. Ecologists are also interested in mating strategies, and the degree of relatedness and kinship bonds and, if all those factors are taken into account, cooperation has to mean rather different things in different contexts. As Riehl (2013) pointed out, when one classifies cooperative behaviour by group composition and social mating system for all known cooperative species worldwide (about 400 species confirmed), then the picture of what cooperation may mean gets rather complicated (see Fig. 1.6).

Fig. 1.6. Different social and kin arrangements of cooperative species. This chart would be thought of as applying to cooperative birds worldwide. (Adapted from Riehl 2013.)
Moreover, it may be important to distinguish between pair-bonded groups and polygamous males (i.e. those that breed with several females) and see whether cooperation has the same importance in the behaviour it is linked to and the consequences it achieves. Some species belong to families in which most species are cooperative and others are not, as is the case in the family of thornbills (Fig. 1.7), yet it seems that brown thornbills do as well as those that act cooperatively. Cooperative behaviour may demand no more than sentinel duties or territorial defence in some species, while in others it may lead to sophisticated patterns of collaboration and communication: the kind that is complex and demands cognitive abilities. In the consequences it achieves, survival of offspring would be foremost on the list of important consequences and another cause, as well as a consequence, might be the evolution of larger brains.

Fig. 1.7. Brown thornbills pair bond but do not raise offspring cooperatively. They are among the very small songbirds in Australia that have been of interest because of their cooperative habits. Little if anything has been said about thornbills as songbirds. Thornbills have beautiful songs and some of the elements are of pure tonal quality and complex trills are part of it. Because complexity of song is generally related to brain size, the investigation of much of this aspect of Australian songbirds is yet to be done. (Photo by John Manger. Sonogram based on recordings by the author.)
The other notable feature of Australian bird life is its longevity and the tendency to pair bond for life. The latter points will feature in the following chapters.
Here it is timely to introduce two important ideas. One is that much of the literature on birds (and other animals for that matter) has emphasised competition in animals. Between species competition may be common but it has no place in pair bonds and may not be relevant. In many circumstances, cooperation may be more common than competition. Competition may sharpen the senses and may be related to physical health and resource security, but it may not necessarily explain cognitive development in birds. So dominant has this view of competition been that the very idea of cooperative behaviour has continued to have its sceptics, popularised by writers such as Richard Dawkins of ‘selfish genes’ (Dawkins 1977). Recent molecular studies have shown that many cooperative groups also contain unrelated individuals and thus the issue is not resolved at all (see also the review by Kaplan 2014). Based on the insistence of several Australian researchers, chiefly Andrew Cockburn of the Australian National University (Cockburn 2006), cooperation is an important topic. It finally opened the door for researching life histories of Australian birds and exploring possible links between cooperation and the evolution of intelligence, as shall become clear in the following chapters.
The second point is that cooperative behaviour, despite its tight definition, does not necessarily mean the same thing in all species and perhaps the time has come to think of cooperation as a more inclusive term. Cooperation may mean assistance that individuals may give to each other or that group members may actively extend to each other. Alternatively, it may mean undertaking a set of activities in which individuals or groups engage together whose outcome is of benefit to the group as a whole.
Recently, Mainwaring and Griffith (2013) argued convincingly that cooperative aspects of social monogamy had been too often overlooked and showed that zebra finches that form strictly monogamous lifelong bonds benefit by one partner acting as a sentinel and an early warning guide while the other is brooding on the nest. Zebra finches have a wide range of natural enemies and the added burden of introduced predators such as cats and rats, but have very few defences against such predators other than vigilance and flight. Hence the warnings by a mate of approaching danger, as their study showed, generally worked to give the brooding mate a chance of escape from the nest at much higher than chance level (Mainwaring and Griffith 2013). In other words, individuals in socially monogamous partnerships may benefit mutually and are more likely to survive when one partner looks after the other in an active way (Zann 1996). Even in this limited form of cooperation, it is clear that ‘sentinelling’ may make a life and death difference. Survival of the pair may mean a chance to renest in the same season. In group contexts, the role of a sentinel is typically on a roster system so that even the sentinel can eventually feed. There is some evidence that well-fed birds are more likely to carry out sentinel duties, at least in Florida scrub-jays (Bednekoff and Woolfenden 2003). Individuals may even help an individual or group belonging to a different species (called heterospecifics), as may happen in shared territories in predator defence.

Fig. 1.8. Apostlebirds belong to the same family as the white-winged choughs and are equally close knit. They live exclusively as a group and always breed cooperatively. These apostlebirds were photographed at Killara Station in the far west of New South Wales.
The range of discussions about cooperation includes also why some species in the same family are pair bonded but do not have cooperative practices (Fig. 1.7), while others do not just cooperate in raising the next generation but do everything together (Fig. 1.8).
Interestingly, in kookaburras the cooperation and the ‘helping at the nest’ has a peculiar twist. Helpers help in order to raise offspring and see them survive to adulthood. Yet, as Legge (2000, 2002) showed, siblicide (the killing of one offspring by another, usually older and stronger one) is not stopped by parents or helpers. Siblicide may happen in almost half of all kookaburra breeding events. Kookaburras may lay up to three eggs that hatch but this may be too many to feed. Legge found that seniors that killed their youngest sibling reached higher asymptotic weights than seniors that did not commit siblicide. In contrast, if the youngest nestling was not killed by its older sibling, but later starved to death, the surviving seniors were skeletally smaller and had retarded feather development compared with seniors from other broods. On the other hand, kookaburra pairs that have not been able to breed are willing and able to adopt orphaned youngsters that have fledged but are still dependent on being provisioned. This latter knowledge is not based on any controlled experiment but on the cumulative experience of decades of hands-on work by wildlife organisations that rehabilitate injured or orphaned native animals and this information is passed on in training sessions about appropriate release procedures for individual species. It is also monitored post release and I know of at least two cases in which convincing evidence of the success of this method was available. It is worth mentioning here because adoption of unrelated juveniles is extremely rare in nature. We know of helping behaviour or even adoptions in groups of primates and in packs of wild dogs in which subordinate females sometimes lactate and help feed the offspring of the dominant female or replace her in case of her death (see Rogers and Kaplan 2001) but this is within packs and established social groups only and does not include youngsters from other packs or groups. That we know of two native bird species (kookaburras and white-winged choughs) that will take in foreign or orphaned juveniles must be considered significant, particularly since both species are also cooperative breeders.
At the most extreme end of the spectrum are cooperatively breeding species that consist of inseparable tight-knit groups in which everything is shared, as is the case in the closely related mud nesters, Corcoracidae, to which apostlebirds (Fig. 1.8) and white-winged choughs belong. White-winged choughs, so Ian Rowley wrote, excel in cooperative behaviour. They forage, drink, bathe and roost together and together mount a defence against intruders or predators. They also raise their offspring together (Rowley 1978). Remarkably, they not only build nests together but also share incubation, guarding and feeding of the young. Subgroups may consist of 2–4 birds but the entire group could be up to about 20 strong. It is puzzling that, given this extraordinary degree of cooperation, they do relatively poorly in raising young successfully. In his study, Rowley observed 186 nesting attempts producing 1–4 offspring, yet, despite the help at the nest and the constant protection, only 16% of those raised made it to sexual maturity, which is noteworthy itself because choughs do not mature sexually until 4 years of age. Only very large groups apparently raise all their hatchlings successfully and then raise them to sexual maturity (Boland et al. 1997a).
It is worth looking a little more closely at the level of cooperation versus their general life history. Indeed, as shall be shown throughout the book, some of the cognitive aspects of Australian bird behaviour might even be based on close bonding and/or group cohesion. Or put another way: cooperation may have been central for the success of birds.
For many Australian birds that will be discussed, survival may depend on cooperation for mutual protection and even sustenance. In Australia, a continent with ancient lineages of living animals and birds, one might even be tempted to speculate, as some writers have done (Nicholls et al. 2000), that, for many birds, cooperation may be the older form of association with conspecifics than competition, if not for most. Nicholls and colleagues investigated thornbills, or rather genus Acanthiza, which contains examples of both cooperatively and pair-breeding species. They tested whether the occurrence of cooperative breeding could be explained in terms of broad environmental factors or life history but found no conclusive link across genus Acanthiza. However, as the pair-breeding Acanthiza species clustered into just two clades, they suggested some influence of phylogenetic history on the occurrence of this different breeding system. Combining the results of this study with other data, they concluded that the tendency to breed cooperatively could be ancestral, not only for the thornbills but for the entire superfamily Meliphagoidea, to which all the honeyeaters, Meliphagidae, also belong (Nicholls et al. 2000).
Cooperation does not always pre-suppose relatedness, as has been suggested before, be this for breeding purposes or for mutual protection. To make a case, I would like to cite an unusual example observed over an 18-year period. Regularly, and over many years I had hand-raised Australian magpie nestlings (Kaplan 2005). When nestlings were of exactly the same age, as we were able to determine quite easily, they were raised together in one nest. That particular year four nestlings were found separately in areas so far apart from each other that relatedness could be ruled out. As they grew and eventually acquired adult plumage, they were identified as all male. Two months after fledging into an outdoor aviary, they were soft-released into the garden of the 5-acre property. The expectation was that the group of four would eventually break up and each join a bachelor flock of which there were several locally. Nothing of the sort happened. The four stayed together and on the property and they could thus be monitored very easily. Magpies have wing patterns that are unique to each magpie and hence readily identifiable. They had adopted the property as their territory and very effectively defended it. Magpies reach sexual maturity after the first moult and first adult plumage (i.e. at the age of 1 year), but they usually do not pair up until the age of 5 years. However, these four did nothing to find a mate. They did not disperse and no one left the property. Eight years passed. One day in winter (July), I happened to stand in the garden with the four males that were very tame and feeding unperturbed together on the ground just a few metres away from me, I saw a lone female fly in, land near the four and gingerly and tentatively approach the group of four. The males briefly turned around but did not stir – it was the briefest of acknowledgements but the decision was unanimous that she could stay. In September, a nest built at the centre of the territory in a pine tree on the property, produced two hatchlings. The four males and the lone female all helped in feeding them and then the two offspring stayed for an entire year before they dispersed, already with adult plumage. The remarkable part is that the group stayed together for a further 10 years and together kept raising offspring successfully. I could never establish who the father might have been but the importance of this example, spanning nearly two decades, is that relatedness was not the driver of their cooperation. It could be argued that this was an artificial situation and the males simply behaved as if they were siblings. Usually, however, siblings do not stay together nor do they admit a partner into the existing group. Still, even though this example may raise new questions, it makes the point that there is a good deal more flexibility in social relationships in birds than had been thought. It has been documented in apostlebirds that immigrants are regularly accepted into their tight-knit groups (as many as 12% in Woxvold and Mulder’s study of 2008), but such acceptance initially does not give the newcomers reproductive rights (Woxvold and Mulder 2008), while the female magpie might have been accepted purely for reproductive reasons.
Cooperation does not imply harmoniousness, although it does imply that there are ground rules at work. There may be compatibility issues of individuals and some birds choose to stay together even though there are tensions, so well titled in Marzluff and Balda’s paper on pinyon jays: ‘making the best of a bad situation’ (Marzluff and Balda 1988). But in some cases, partners split up. ‘Divorces’, even in long-bonded pairs, do occur and extra-pair matings occur in many social species. Both are well documented in the cooperatively breeding superb fairy-wren (Cockburn et al. 2003) and the cooperatively breeding white-browed scrubwren (Whittingham et al. 1997). Partner changes may occur for a number of reasons. In cockatiels it has been shown that low reproductive success in pairs can lead to extra-pair matings and eventually to separation. This extra-pair copulation led to mate switching and to reproductively more successful relationships (Spoon et al. 2006). This result suggests that extra-pair sexual behaviour facilitates mate switching in cockatiels, and the authors also suggested something quite important that might go unnoticed: behavioural compatibility in a couple has been identified as an important variable in several species (e.g. in pinyon jays as cited above) and in cockatiels. Indeed, in cockatiels it could make or break the bond (Spoon et al. 2006). This goes to the heart of our understanding of animals. The general view was that any male or female when put together would automatically reproduce. While we know from research that, in some species, females carefully select males for breeding, the possibility of compatibility (even affection) had been thought to be implausible and was once condemned as anthropomorphic.
Overall, then, there are a number of very compelling reasons to speak about Australian birds as very precious, special and puzzling. The history of the continent, its vastness and arid expanses, the changes in climate and in sea levels and its isolation have led to conditions for species to evolve in very specific and special ways and, in the process, many of them as shall be shown have also excelled in cognitive abilities.