Chapter Eighteen
INTRODUCTION
Seagrasses have a wide distribution throughout the world's oceans, being absent only from the polar seas, although the genera Phyllospadix and Zostera are present as far north as the Bering Sea and as far south as the Tasman Sea. From the cold subpolar regions to the equator, seagrasses inhabit a variety of shallow-water coastal habitats where they play a key ecological role (Hartog 1970). These marine phanerogams (i.e., seed-bearing plants) are well-known components of temperate intertidal 'salt-marsh' communities (e.g., Zostera marina), recognized for their ability to help stabilize coastlines and to provide food and shelter for a host of marine organisms (Chapman 1974). Throughout their distributional range, sea-grass systems are considered as important nursery grounds for many economically important marine finfish and shellfish species. Of particular concern to Indonesia is the fact that a number of commercially valuable penaeids (e.g., Penaeus esculentus, P. semisulcatus and Metapenaeus ensis) are dependent on seagrass communities for food and protection during at least one stage of their life cycle (Coles et al. 1985; Staples et al. 1985). The existence of productive commercial shrimp fisheries in the coastal waters of the Am Islands, Moluccas, as well as in the Gulf of Carpentaria, is largely due to the presence of extensive seagrass beds in these areas. For example, Suwartana (1986) reported an average shrimp catch of about 490 tons per year from commercial trawling grounds south of the Aru Islands. Destruction of seagrass habitats by trawlers will ultimately bring an end to the shrimp fishery in the area. In addition to their economic importance, seagrass ecosystems are critical habitats for a number of endangered marine species (e.g., Dugong dugon and Chelonia mydas)and support a rich and diverse fauna and flora.
Throughout the Indonesian Archipelago, seagrasses are often characteristic features of soft-bottom habitats (i.e., sandy bays, mud flats, lagoons), where they often form extensive mixed or monospecific meadows (fig. 18.1). Mixed seagrass communities composed of 8-9 species are common in many coastal areas in Indonesia (e.g., Lombok, Lembata Island, etc.), a unique feature that sets them apart from the monospecific seagrass communities characteristic of the Caribbean and many subtropical and temperate regions. Along coastlines dominated by mangrove forests, seagrass communities often provide a functional link and a buffer between the seaward coral reefs and the inshore mangroves (fig. 18.2). The functional linkages between these three shallow-water tropical coastal ecosystems have attracted considerable research interest. The term ecosystem as often applied to seagrasses is somewhat problematic (Basson et al. 1977; den Hartog 1980; IUCN 1987; Phillips and Menez 1988; Sheppard et al. 1992). The difficulty stems from the fact that seagrass communities vary greatly in areal dimension (from a few m2 to thousands of m2) and in their association with other ecosystems.


Figure 18.1. Seagrasses are found in a variety of shallow-water coastal as well as offshore habitats where they form extensive monospecific or mixed meadows. A) Monospecific meadow of Cymodocea rotundata. B) Mixed seagrass community consisting of T. hemprichii, Enhalus acoroides and Cymodocea rotundata. Salabanka Islands, Southeast Sulawesi.
Photos by Tomas and Anmarie Tomascik.

Figure 18.2. Seagrass meadow at Pulau Rinca, East Nusa Tenggara.
Photo courtesy of A. Ibrahim, P30-LIPI, Jakarta.
Seagrasses are frequently associated with coral reefs (i.e., fringing, barrier, patch and atoll reefs), where they occupy a variety of intertidal and subtidal habitats. However, they are most often found, and achieve their highest densities and biomass, in shallow-water back-reef environments (reef flats, moats) and lagoons. On some sheltered, but current-swept, barrier reefs and atolls, some seagrasses can even dominate the reef crest. While seagrasses are often viewed as growing mainly on soft substrata, Thalassodendron ciliatum is often found attached to hard rock and coral limestone. On a number of barrier reefs and atolls in the Banggai Islands, T. ciliatum is a key component of the subtidal seaward reef crest. Masses of decaying T. ciliatum leaves cover the seaward reef slopes to depths in excess of 45 m, and large schools of juvenile Siganus spp. feed along the seaward edge of the reef, where T. ciliatum forms luxuriant monospecific meadows (fig. 18.3). From an ecological perspective, it seems that in these instances it is perhaps more appropriate to view seagrasses as reef-associated communities. Admittedly, a mixed seagrass community of 5-7 species occupying a 5-km-long and 200-m-wide fringing reef moat may be considered as an ecosystem (e.g., west coast of Lembata Island, Nusa Tenggara). Based on their structural and functional characteristics, large seagrass meadows (i.e., 10s of km2) along many coastlines (e.g., Miskam Bay, Sunda Strait; Banten Bay, southwest Java Sea) and in shallow-water inter-reef areas (e.g., Am Islands, Tanimbar Islands, etc.) may be viewed as ecosystems.

Figure 18.3. Seagrasses, such as Thalassodendron ciliatum, are integral components of Indonesian reefs. A) T. ciliatum forms extensive meadows along the seaward edge of many barrier reefs and atolls in the Banggai Islands. B) High secondary productivity of many barrier reefs and atolls in Indonesia may be attributed partially to the significant export of seagrass leaves into the deeper parts of the seaward reef slope. On the Pulau Sago barrier reef, decomposing leaves of T.ciliatum were found to depths of 30 m or more.
Photos courtesy of Coral Cay Conservation, Ltd.
SEAGRASS ORIGINS
Seagrasses are among the few plants that have migrated back to the sea, and as they did, they brought with them the evolutionary heritage of their terrestrial existence (Raven 1977; King et al. 1990). Based on evidence of two fossil genera, viz. Archeozostera and Thalassocharis, den Hartog (1970) suggested that the return to fully marine existence occurred roughly 100 million years ago during the Cretaceous. Since angiosperms appeared sometime in the late Jurassic (c. 150 Ma B.P.), or early Cretaceous (144-97 Ma B.P.), it appears that seagrasses diverged early from the mainstream angiosperm (i.e., flowering plants) evolution (Larkum and den Hartog 1989). Seagrasses arose either from freshwater hydrophytes or from marsh-type plants (den Hartog 1970). The fossil evidence suggests that by late Cretaceous seagrasses were well established throughout the shallow waters of the Tethys Sea, where speciation occurred (Brasier 1975; Mukai 1993). Dispersal of seagrasses to other regions progressed rapidly since the Eocene (Larkum and den Hartog 1989). Assemblages of benthic organisms associated with seagrass beds are characteristically different from those in adjacent habitats (Hutchings 1981). This is very useful knowledge to paleontologists, since seagrasses in general do not fossilize well, while associated foraminiferan and gastropod shells do. Brasier (1975) showed that there is a strong association between a number of shallow-water foraminiferans and seagrass beds (e.g., Sorites marginalis with Thalassia spp.). A number of other faunal groups (e.g., gastropods and marine mammals) have also been used as evidence for the existence of ancient seagrass beds (Domning 1977, 1981; Heck and McCoy 1979). Seagrass-associated foraminiferan faunas have been used as evidence for the early existence of seagrass beds in the Tethys Sea, Caribbean and North Atlantic (Wright and Murray 1972; Brasier 1975; Eva 1980).
Two opposing hypotheses have been proposed to explain the modern distribution of seagrasses, namely 1) vicariance hypothesis; and 2) centre-of-origin hypothesis. The vicariance hypothesis, as proposed by McCoy and Heck (1976), is based on plate tectonics, climate change, as well as ecological considerations such as extinction and species-area relationships. Based on the distribution of modern corals (scleractinians), seagrasses and mangroves, McCoy and Heck (1976) concluded that: "…biogeographical patterns are better explained by the existence of a previously widely-distributed biota which has since been modified by tectonic events, speciation, and extinction, in accordance with modern geological and biogeographical theory". On the other hand, the centre-of-origin hypothesis maintains that modern distribution patterns of seagrasses may be explained by their dispersal by radiation from a region of highest diversity, which is considered to be the centre of origin (den Hartog 1970). It was proposed that "Malesia" (comprising the Indonesian Archipelago, Malaysian Borneo, Papua New Guinea and northern Australia) is such a centre of origin for modern-day seagrasses (Larkum and den Hartog 1989). Note that "Malesia" is not to be confused with Malaysia, a nation in Southeast Asia. Considering the Cretaceous age of seagrasses, and the recent geologic evolution of the Indonesian Archipelago, the hypothesis that this region has been the "centre of origin" is highly unlikely (Larkum and den Hartog 1989). However, considering the fact that oceanic plates in the central Indo-west Pacific have been roughly in the same geographic position at least since the late Miocene (c. 10 Ma B.P.) (Hall 1995), it is probable that "Malesia" has acted as a "modern-day" centre of origin for a wide range of marine flora and fauna, including seagrasses. Mukai (1993) has demonstrated that modern distribution patterns of seagrasses in the western Pacific are indeed a function of oceanic currents and the distance from the "modern-day" centre of origin (i.e., Malesia). His data illustrate that, as one follows the major oceanic currents further away from the centre of high diversity in Malesia, there is a progressive loss of diversity, with peripheral regions (i.e., Japan, south Queensland, Fiji) having the least number of tropical seagrass species. Note, however, that Mukai's (1993) analyses of seagrass distributions along the north-flowing Kuroshio and the south-flowing east Australian currents also reflect strong latitudinal (i.e., temperature) gradients. Mukai (1993) pointed out that distribution of seagrasses along these gradients is also under temperature influence.
Long-distance dispersal is central to the centre-of-origin theory, and has often been criticized for being generally unsubstantiated for corals, seagrasses or mangroves (McCoy and Heck 1976). It is generally accepted that modern seagrasses have poor means for long-distance oceanic dispersal, since their sexual propagules lack the basic adaptation of buoyancy (Opurt and Boral 1964; McCoy and Heck 1976; Larkum and den Hartog 1989). However, the thousands of islands in the central Indo-west Pacific may have served as "stepping-stones" (Veron 1995) in their dispersal and speciation, thus their apparent deficiency for long-distance propagules may not be a major block to their wide dispersal. As Mukai (1993) pointed out, sea-grasses have a number of alternative means for dispersal other than sexual propagules. Extensive seagrass "rafts", up to a few kilometres long and hundreds of metres wide, are a frequent site in the Indonesian archipelagic seas. In the Banggai Islands, for example, long seagrass "rafts" (mainly Thalassodendron ciliatum) were observed to link neighbouring islands. Floating among the dead seagrass material (i.e., detritus) are thousands of seagrass fragments with viable roots and rhizomes that are able to take root if suitable substrate becomes available. This mode of dispersal has been shown to occur elsewhere (Pointer et al. 1989). The role of large vertebrate herbivores (Chelonia mydas and Dugong dugon) in seagrass dispersal has thus far not received serious attention (Mukai 1993).
It needs to be acknowledged, however, that, while oceanic currents are central to the dispersal of marine organisms, and thus to the centre-of-origin theory, they also form effective geographic barriers, and the Indonesian Archipelago is an excellent example. High-velocity currents (e.g., >5.0 m.sec-1; e.g., Sape Strait) flowing between the islands through numerous narrow straits (usually in a north-to-south direction) may explain some longitudinal differences in species composition of shallow-water benthic communities, especially along the Great Sunda Arc.
BASIC CLASSIFICATION
Seagrasses flower, pollinate, produce fruit and disperse seeds as do many terrestrial grasses. In fact, the classification of seagrasses is based on floral and related characters (King et al. 1990). In addition, the tropical genera are morphologically distinct, thus species separation is possible on the basis of morphological and anatomical features alone (Appendix I).
Seagrasses are marine monocotyledons with well-developed rhizome-root systems belonging to the single Subclass Alismatidae (Magnoliophyta: Liliopsida) (Kuo and McComb 1989; King et al. 1990). Note that there are a number of classification systems for plants. The Subclass Alismatidae unites two former subclasses, the Holabiae and Fluviales. In alternate classification systems, seagrasses are placed in the Subclass Monocotyledoneae in Class Angiospermae. Of the four recognized families, two families occur in Indonesian waters, namely the Hydrocharitaceae and Cymodoceaceae. The Family Hydrocharitaceae contains predominantly freshwater plants, while Cymodoceaceae, Posidoniaceae and Zosteraceae are entirely marine (King et al. 1990). Seagrasses are the only rooted vascular flowering plants adapted to live fully in the marine environment. Their marine existence is possible through a number of key adaptations that includes high salt tolerance, the ability to send roots into the substrate for anchoring, as well as the ability to grow and reproduce while submerged (Arber 1920). Seagrasses are characterized by the absence of stomata, retention of a thin cuticle, schizogenous development of the lacunar system and the presence of diaphragms in the lacunar system (Larkum et al. 1989). One of the most important reproductive adaptations is their ability for hydrophilous (i.e., underwater) pollination.
Morphological/Anatomical Features
Seagrasses, in general, are similar in external appearance, but exhibit considerable diversity in the structural characteristics of their vegetative organs (fig. 18.4). Being true vascular plants, seagrasses also exhibit structural and functional similarity to their distant terrestrial relatives, the grasses (Lanyon 1986). In contrast to benthic marine algae (i.e., seaweeds), seagrasses have true roots, leaves, and an internal lignified vascular transport system for nutrients, water and gases (Fortes 1990a).
Roots. There are distinct morphological and anatomical differences in root structure among the seagrasses, which is a useful taxonomic feature. In species such as Halophila and Halodule the roots are fragile, hair-like, small-diameter structures, while in Thalassodendron the roots are tough and woody, with lignified epidermal cells. In comparison to terrestrial plants, the roots and root hairs of seagrasses are less developed. Nonetheless, numerous studies have shown that the roots and rhizospheres of seagrasses are functionally similar to those of terrestrial plants (McRoy and Barsdate 1970; Smith et al. 1979; Boon et al. 1986).
The adventitious roots arise from the lower surface of rhizomes, and show numerous specialized adaptations (e.g., aerenchyma, thin unlignified epidermal cell) to aquatic environment (Arber 1920; Sculthrope 1967). All roots have a central stele surrounded by endodermis. The stele contains well-developed phloem (nutrient transport tissue) and very weakly lignified xylem (water-conducting tissue) (King et al. 1990). Since seagrass roots have weakly developed water-conducting tissue, it has been suggested that they play an insignificant role in water uptake (Tomlinson 1969). However, others maintain that all structures of the vascular bundles are involved in transport of materials (Roberts et al. 1985): Patriquin (1972) demonstrated that seagrasses are able to absorb nutrients from the interstitial water through their root-rhizome system. Furthermore, nitrogen fixation by heterotrophic bacteria in the rhizosphere of Halophila ovalis, Enhalus acoroides, Syringodium isoetifolium and Thalassia hemprichii has shown to be substantial, up to 40 mg N.m-2.day-1 (Moriarty and Boon 1989). In addition to nitrogen fixation, rhizosphere bacteria play an important role in converting insoluble inorganic phosphate into soluble phosphate and in deaminating amino acids to yield ammonia (Roberts 1993). Bacterial colonies found in the seagrass rhizospheres may apparently play an active role in nitrogen fixation and nutrient uptake by the roots (Kuo 1993). Nitrogen fixation is an important process since nitrogen is an essential metabolite used primarily as a structural component of cells.
Seagrasses are frequently found in shallow intertidal coastal habitats where muddy sediments, rich in organic matter (derived partly from terrestrial sources), often become anoxic. In sheltered environments with insufficient circulation (currents and waves), these seemingly inhospitable conditions (high temperatures, anoxia, subaerial exposure, etc.) often support extensive seagrass meadows. Anoxic conditions in sediments, however, liberate large quantities of phosphate (an essential metabolite involved directly in the energy cycle of cells) that are readily absorbed by the seagrass roots and transported to areas of growth. Their success in these habitats is related partly to roots and rhizomes which have evolved specialized anatomical as well as physiological features to cope with anoxia.
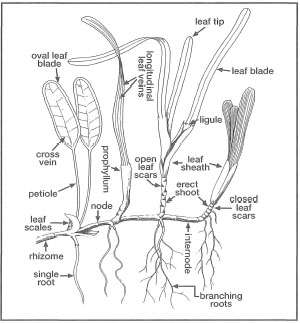
Figure 18.4. General morphology of seagrasses. The seagrass illustrated is a "composite" drawing of a number of different species to illustrate key morphological features that are the basis of seagrass taxonomy.
From Lanyon 1986.
Among their many functions, seagrass roots serve as reservoirs for photosynthetically generated oxygen that is transported to them from the photosynthetically active epidermal layers of blades via diffusion through an intricate lacunal (air) system (Sand-Jensen et al. 1982; Larkum et al. 1989). Most of the oxygen stored in roots and rhizomes is used internally during basic metabolism of cortical and epidermal cells as well as by the microflora of the rhizosphere (Kuo et al. 1981). Some seagrasses are known to release oxygen from their roots (e.g., Halophila ovalis), while other species (e.g., Thalassia testudinum) seem to do better under more anoxic conditions. Larkum et al. (1989) point out that the transport of oxygen to the roots is just sufficient to meet the metabolic requirements of the root epidermal cells and their associated microflora. Bristow (1975) and others have suggested, that through their root/rhizome system, seagrasses are able to modify the surrounding sediment environment through transport of oxygen and other chemical compounds. It has also been demonstrated that seagrasses are able to modify lacunal systems according to the degree of anoxia existing in the sediments (Pen-hale and Wetzel 1983). It has been suggested that the release of oxygen to the sediments is a detoxifying function, similar to that in terrestrial plants (Roberts 1993). This ability seems to be an adaptation for anoxic conditions that frequently exist in silty/muddy sediment substrates.
Since seagrass roots are sites of active metabolism (i.e., respiration), it is probable that CO2 concentrations in the root tissue are relatively high. Whether the lacunal system also transports some of the metabolically derived CO2 into sites of photosynthesis in the leaves, is yet to be determined. Streams of oxygen and/or CO2 bubbles are frequently seen when roots and rhizomes are cut.
Rhizomes and Stems. All seagrasses possess more-or-less cylindrical rhizomes which are mainly herbaceous, although in Thalassodendron ciliatum the sympodially branching rhizomes are very woody, which enables the species to inhabit a variety of reefal habitats where other seagrasses are not able to survive. Its ability to attach to solid substrates has allowed T. ciliatum to colonize high-energy spur-and-groove zones of fringing reef along the south coast of Bali, where they are exposed to the full force of the Indian Ocean swell, yet they are remarkably difficult to remove.
The structure of rhizomes and stems is highly variable among the seagrasses, as is the arrangement of vascular bundles in the stele (den Hartog 1970; Tomlinspn 1982). Rhizomes, together with roots, anchor the plant to the substrate. Rhizomes are often buried under sediments where they can form extensive networks. They are the primary means for vegetative propagation (Tomlinson 1974). It has been suggested that maintenance of seagrass meadows through vegetative propagation is more important than seed production, which serves as the primary means for dispersal (Tomlinson 1974). The rhizomes constitute about 60%-80% of seagrass biomass (Moriarty and Boon 1989), and in Enhalus acoroides they are covered by a thick covering of fibrous remains of old leaf sheaths, which add considerably to their bulk.
Leaves. As in all monocotyledons, seagrass leaves are produced from basal meristems (i.e., growing tips) located at the apices of the rhizome and its branches (Lanyon 1986). In spite of the general similarity in form, seagrass species exhibit specific morphological and anatomical features that are of great taxonomic value (Tomlinson 1980). Some of these morphological features easily seen by a naked eye are the pattern of leaf venation, shape of the leaf apex and the presence or absence of ligules (fig. 18.4). For example, the leaf apex of Cymodocea serrulata is rounded and clearly serrated, while that of C. rotundata is flat and smooth. Seagrass leaves have two distinct parts, the sheath and blade. The non-chlorophyllous sheath, or leaf base, encloses the growing tip of the rhizome and protects the young leaves. However, genus Halophila with petiolate leaves has no leaf sheath. In all other seagrass species the leaves are elongated or ribbon-like with an ensheathing leaf base (fig. 18.4).
The unique anatomical feature of seagrass leaves is the absence of stomata and the presence of thin cuticle (Tomlinson 1980; Kuo 1983). However, Tomlinson (1982) reported apparently nonfunctional stomata in Thalassia testudinum in the Caribbean. The thin cuticle offers low resistance to ion movement and carbon diffusion so that the leaves may absorb nutrients directly from ambient seawater. The seawater provides the plants with an abundant source of bicarbonate for inorganic carbon used in photosynthesis (fig. 18.5). The major pathway of inorganic carbon across the thin cuticle is via CO2, which may explain the high rates of productivity (Larkum et al. 1989). Since seagrasses are aquatic plants, they require no protection against desiccation, and the cuticle in fact offers none, due to its porous and thin structure. In intertidal habitats where seagrasses may become periodically subaerially exposed during low tides, desiccation is apparently avoided by leaves overlapping one another on the wet substrate, which maintains high relative humidity (King et al. 1990).
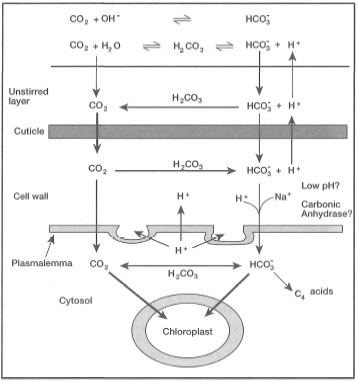
Figure 18.5. Illustration of theoretical movements of HCO3/CO2 across epitaxial face of the epidermal cell of seagrass leaves.
Redrawn from Larkum et al. 1989.
The chloroplast-rich epidermis of seagrass leaf blades is the major site of photosynthesis. In Enhalus and Thalassodendron the epidermis is uniformly chlorenchymatous but contains tannin, while in Syringodium, Halodule, Cymodocea, Thalassia and Halophila the epidermis contains chlorenchymatous as well as large tannin secretary cells (Kuo and McComb 1989). In addition to the chloroplasts, the epidermis contains a variety of other organelles such as mitochondria, endoplasmic reticulum, dictyosomes and lipid droplets. The outer walls of epidermal cells are thickened, which adds to the structural strength of the blade, however, they are not lignified, (Kuo 1978). The surface shape of epidermal cells differs between species and is frequently used as a taxonomic tool.
The unique anatomy of seagrass leaf tissue allows for rapid export of photosynthetically derived oxygen into the seawater. This can be seen in the field, by the presence of numerous gas bubbles that are frequently attached to the blade surfaces (fig. 18.6). In extensive seagrass meadows gas bubbles are frequently seen rising to the surface through the water column. Some of the photosynthetically derived oxygen is also discharged directly into the mesophyll layer, which contains numerous air spaces or lacunae, that may have a number of functions. The lacunal system transports oxygen to rhizomes and roots through diffusion, and serves as a temporary reservoir in gas exchange. The lacunae may also play a role in buoyancy, since seagrasses lack the fibrous support system typical of land grasses. The mesophyll cells are usually thin-walled and highly vacuolate. Unlike in terrestrial plants, mesophyll cells in seagrasses have only a few chloroplasts.

Figure 18.6. During high rates of photosynthesis excess oxygen produced in the photosynthetic epidermal layers is rapidly discharged into the surrounding seawater. Cymodocea rotundata, Sanur Lagoon, Bali.
Photo by Tomas and Anmarie Tomascik.
Sexual Reproduction
Seagrasses colonize new areas by dispersal of sexually-produced propagules. The majority (c. 70%) of seagrasses are dioecious (separate sexes) a compared to 7.6% for all angiosperms. The dioecious mode of reproduction may reflect an altered pattern of resource allocation for male and female functions (McConchie and Knox 1989). Morphologically and functionally, the pollen and stigmas of seagrasses differ markedly from terrestrial angiosperms. In many freshwater angiosperms, flowers are borne above-water and pollination is aerial. Seagrasses utilize three modes of pollination. The most common is hydrophilous pollination, where the pollen is released directly into the water column to be dispersed by currents. Most seagrass species have waterproof pollen and stigma, while others rely on tidal cycles to avoid water contact (McConchie and Knox 1989). Another mode is ephydrophily, where the pollen dispersal occurs at the surface. Enhalus acoroidesis unique among seagrasses, since it is the only species with subaerial pollination. However, the pollination process is controlled by the tidal cycle (King et al. 1990). In this mode the entire male flower breaks free and rafts the pollen to the receptive female flower (McConchie and Knox 1989). Pollen in seagrasses is either spherical (e.g., in Hydrocharitaceae) or filiform (e.g., in Cymodoceaceae), but both forms share similar features, such as reduced wall layers and the absence of germination apertures (McConchie and Knox 1989). Dispersal is facilitated in some species by viviparous seedling (e.g., Thalassodendron). Once established, seagrasses spread vegetatively by horizontal, underground rhizomes which, with the roots, can form dense networks in the substrate.
Studies on seagrass reproduction in Indonesian waters are rare. One of the most comprehensive data sets on the flowering and fruiting of seagrasses is from the Spermonde Archipelago (Verheij and Erftemeijer 1993). During a three-year study, information on the timing of flowering and fruiting of six species of seagrasses was collected (table 18.1).
A number of environmental factors, such as tides, sea surface temperatures and day length, are known to influence floral induction in seagrasses (Pettitt 1984; McConchie and Knox 1989). The data presented in table 18.1 were collected over a period of three years (Verheij and Erftemeijer 1993), and are considered to be representative of the yearly flowering and fruiting cycle of seagrasses in the Spermonde Archipelago. The data indicate four reproductive patterns.
Enhalus acoroides does not show any reproductive seasonality, since flowering and fruiting was observed every month of the year. Thalassia hemprichii shows a similar pattern, with the exception that flowering and fruiting is interrupted during January, June and July. The seemingly continual reproductive activity of E. acoroidesis somewhat surprising, since the area is influenced by a strong monsoonal climate (fig. 18.7) as well as by mixed, dominant diurnal tides (fig. 18.8). In contrast, Fortes (1990) reported that in the Philippines, Enhalus acoroides flowers from late April through late August. The timing suggests that in the Philippines flowering is related to environmental conditions (i.e., day length, temperature and rainfall). Fruiting in E. acoroides was reported to occur during the latter half of the flowering period (peak in July) corresponding to the longest day lengths and heaviest rainfall (Fortes 1990a). Germination takes place in August. Interestingly, Fortes (1990) reported that the greatest biomass accumulation (i.e., growth) inE. acoroides occurs from September through December, when environmental conditions appear to be the most stressful (i.e., frequent low tides, long subaerial exposures). In contrast, Erftemeijer (1993) demonstrated that the lowest biomass in E. acoroides occurs during periods when low tides correspond to daylight hours, which occurs between August and January. For example, leaf-blade biomass of E. acoroides in the Spermonde Archipelago peaks during May -July (fig. 18.9).
Table 18.1. Phenological overview (flowering and fruiting) of six seagrass species from Spermonde Archipelago, South Sulawesi over a one-year period. The dashed lines indicate flowering and/or fruiting periods.
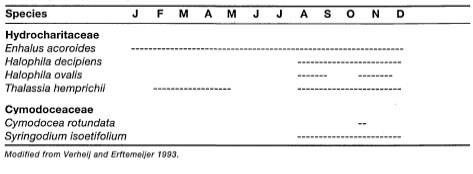
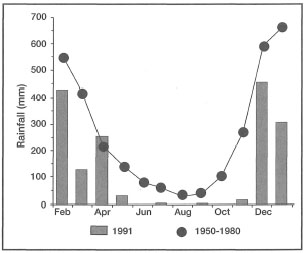
Figure 18.7. Average monthly means for rainfall (mm) during 1991 (grey bars) and average monthly rainfall based on records from 1950 through 1980 (black circles).
From Erftemeijer 1993.
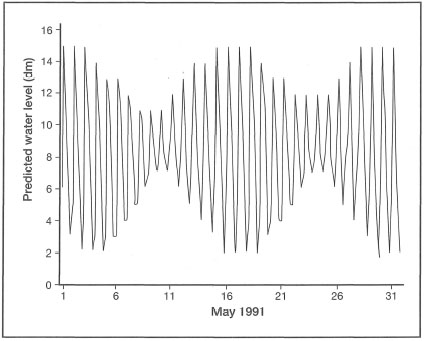
Figure 18.8. Predicted mixed, dominant diurnal tide at Ujung Pandang, South Sulawesi; (F=K2+O1/M2+S2=2.37).
From Erftemeijer 1993.
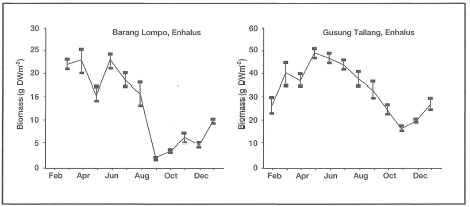
Figure 18.9. Seasonal fluctuations In leaf-blade biomass (g DW m-2) of Enhalus acoroides at Barang Lompo (reefal habitat) and Gusung Tallang (coastal habitat) during 1991. Values are monthly averages ±SD.
From Erftemeijer 1993.
Distinct reproductive seasonality is exhibited by Halophila ovalis, H. decipiens and Syringodium isoetifolium. In these three species, reproductive activity occurs between August - December when environmental conditions appear to be the most stressful. From August through December (Southeast Monsoon) low spring tides occur during daylight hours, which exposes the intertidal seagrass communities to considerable stress. Indeed, above-ground biomass production in E. acoroides and Thalassia hemprichii are significantly reduced due to intense midday insolation, high temperatures, as well as severe desiccation and burning (Erftemeijer 1993). It is interesting that flowering and fruiting in Halophila and Syringodium seem to occur precisely when the biomass of their main competitors is at its lowest. The flowering and fruiting of Cymodocea rotundata, restricted to one month, is difficult to explain, however, as Erftemeijer (1993) points out, the years 1990-1993 were strong El Nino years, associated with higher sea surface temperatures in many parts of the world. It is possible that fruiting and flowering in C. rotundata is more sensitive to temperature anomalies than in the other seagrasses.
Long-term reproductive studies from other parts of the archipelago are lacking. Brouns (1985) reported that in the Banda and Flores Seas (i.e., Kaledupa and Taka Bone Rate Atolls, respectively), Thalassodendron ciliatum was reproductively active from September through October, when sampling was conducted during the Snellius II Expedition. A large percentage of primary shoots at Keladupa (54%) and Taka Bone Rate (23%) had almost-matured fruits. The discrepancy between the two sites, in terms of percentage of primary shoots with fruits, is probably due to different sampling dates (Brouns 1985). Seagrasses sampled in mid-October were in the initial stage of germination (Brouns 1985).
SEAGRASSES OF SOUTHEAST ASIA
The species composition of the seagrass flora in the ASEAN region is largely a function of the intensity and extent of sampling. —FORTES 1990B
The greatest diversity of seagrasses occurs in the Indo-Pacific region. In his seminal publication of The Seagrasses of the World, den Hartog (1970) recognized 49 species of seagrasses belonging to 12 genera, six subfamilies and two families. His work generated the impetus for research in this field, and, as a result, considerable progress has been made in our understanding of these systems during the past 20 years. The species number alone has increased to 58 worldwide, with all new additions coming from Australia (Kuo and McComb 1989). In fact, Australia, with 31 seagrass species and 11 genera, is the most diverse seagrass province in the world, and with 20 seagrass species, Western Australia is the most diverse single region (Fortes 1992).
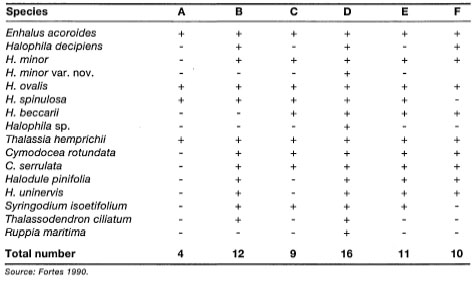
The exceptionally high seagrass diversity in Australia is mainly due to the large number of subtropical and temperate groups (e.g., Amphibolis, Heterozostera, Posidonia) that are not found in equatorial regions. The role of temperature in the distribution of marine algae is well-known (Setchell 1915), and applies to the seagrasses, as well (Setchell 1920,1935). Out of the nine species of Posidonia, eight are endemic to Australia, with P. oceanica being restricted to the Mediterranean (Kuo and McComb 1989). In comparison, the tropical Great Barrier Reef has 14 tropical seagrass species, which is lower compared to the Southeast Asian region as a whole. The Southeast Asian waters (ASEAN) support a relatively diverse assemblage of seagrasses, with 16 species from seven genera (table 18.2).
Paying heed to comments by Fortes (1990b), the data presented in table 18.2 need to be interpreted with caution, especially with regards to the apparently absent species within each ASEAN locality. As was pointed out by Fortes (1990b), Enhalus acoroides, Halophila ovalis, Thalassia hemprichii, Cymodocea rotundata and Halodule uninerois have a broad distribution throughout the ASEAN. While a number of these species (e.g., E. acoroides) are known to form extensive monospecific meadows, they frequently occur in mixed communities of 3-4 species. More limited in their distribution are Cymodocea serrulata, Halodule pinifolia, H. minor and Syringodium ciliatum. Fortes (1990b) suggested that their limited distribution is related to their requirement for specific microhabitat conditions. Based on the rather limited distribution data of Phillips and Menez (1988), Fortes (1990b) suggested that the seagrass flora of the Indo-west Pacific exhibits a rather disjunct distribution pattern. This appears to be true for some species (e.g., Thalassodendron ciliatum), however, the distribution of Halodule uninerois and Syringodium isoetifolium can hardly be considered disjunct, since they are widespread from the west Indian Ocean (Madagascar to Arabian Gulf) to the central Indo-Pacific (ASEAN) and western Pacific (maps of Phillips and Menez 1988). Halophila ovalis, being a rather eurythermic species, has a wide latitudinal distribution in the Indo-Pacific, extending from Japan to southern Australia and from the Red Sea to South Africa. In fact, most tropical seagrasses in the Indo-Pacific have a wide distribution, seven species have restricted distribution (e.g., Ruppia maritima, Halodule pinifolia, Halophila stipulacea, Halophila beccarii, Halophila minor var. nov. and Halophila sp., Halophila spinulosa), and only Thalassodendron ciliatum can be considered as having disjunct distribution (Larkum and den Hartog 1989).
Fortes (1990) suggested that throughout the ASEAN region seagrasses are rather rare in the upper littoral. In the Indonesian Archipelago, seagrass communities are often found in the back-reef areas of intertidal reef flats, where they occupy shallow pools barely covered by seawater. On the other hand, they have often been observed totally dry (burned) during low tides, and thus the generalization that eulittoral habitats uncovered by most tides are devoid of seagrasses is not universally applicable. Fortes (1990b) considers Halodule unineruis (narrow-leaf variety) as an exception to the above generalization; however, mixed intertidal (i.e., frequently subaerially exposed) seagrass communities in the Indonesian Archipelago are a common occurrence. High-velocity tidal currents and wide tidal range are two environmental factors that seem to be characteristic of areas where seagrass communities occur in intertidal reef habitats. Halodule ovalis is often the most abundant species in mixed intertidal back-reef seagrass communities. It seems that desiccation stress may have a role in the establishment of some mixed species communities in the intertidal. Desiccation and high temperature stress may reduce the competitive fitness of dominant species (e.g., Thalassia hemprichii), thus creating favourable conditions for the establishment of high-diversity mixed seagrass communities.
Based on the dominance of the most abundant seagrass species, Fortes (1990b) identified three general zonation patterns in the lower littoral and adjacent sublittoral in the ASEAN region. The Halodule unineruis (narrow-leaf) zone is frequently exposed during low tides (depth range -0.4 to 0.7 m), and is characterized by stable, predominantly fine sand substrates overlying soft to compacted mud. 'Zone two' consists of Halophila - Halodule unineruis association, which forms the upper fringe of the main seagrass bed (depth range 0.2-2 m). Being under tidal influence, the substrate is less stable, and more opportunistic species such as Halophila ovalis tend to dominate. 'Zone three' consists of the Thalassia-Cymodocea-Enhalus association. This association is found in a variety of habitats and under a wide range of environmental conditions. Viewed from successional dynamics, the Thalassia - Cymodocea -Enhalus association is considered to be the "terminal" stage (Fortes 1990b).
Table 18.2. Distribution of seagrass species in the ASEAN (Association of Southeast Asian Nations) region. A: Brunei Darussalam; B: Indonesia; C: Malaysia; D: Philippines; E: Singapore; F: Thailand. '+' indicates record; '-' indicates absence.
THE SEAGRASSES OF INDONESIA
General Distribution
While information on the ecology and biology of Indonesian seagrasses has grown considerably within the past few years (see Kiswara 1994a for review), vast areas of the archipelago (e.g., north coast of Irian Jaya; southwest coast of Sumatra, etc.) have yet to be studied. With 12 seagrass species and seven genera, Indonesian seagrass diversity is surprisingly low, even though habitat diversity is among the highest in the world (table 18.3). The low species diversity may be partly related to the relatively homogenous seawater temperatures throughout the archipelago, and the dominance of the tropical genera, although Halophila ovalis is rather eurythermic (Larkum and den Hartog 1989).
It should be noted that Halophila beccarii may also be present in Indonesian waters, however, it has not been formally recorded as such (den Hartog 1970; Soegiarto and Polunin 1981; Kiswara and Hutomo 1985). Among the 12 species, Thalassodendron ciliatumwas believed to have had limited distribution in the eastern part of Indonesia. However, the seagrass has recently been found on fringing reefs in the Riau Archipelago, west Java Sea (W. Kiswara, pers. comm.). Two other species with relatively limited distribution are Halophila spinulosa and H. decipiens. The diversity of seagrasses is low when compared to some 800 species of marine algae (seaweeds) in Indonesia (Soegiarto and Polunin 1981). With the current database, it is not possible to conduct any meaningful biogeographic studies in the archipelago, since the absence of species at various locations is most likely indicative of sampling effort, and not related in any way to environmental or ecological factors (fig. 18.10). Preliminary broad geographical seagrass distribution maps have been prepared, but many areas of the country remain undocumented (fig. 18.11). The data illustrate that seagrasses occur in all regions of the archipelago, covering an estimated area of about 30,000 km2, yet no endemic species have been found (Nienhuis 1993).
During our surveys, seagrasses were found along mangrove coastlines, estuaries, shallow embayments, an anchialine lagoon of an uplifted atoll, as well as in inter-reef and offshore island situations. In Indonesia, seagrasses are an important component of coral reef ecosystems, found in habitats extending from the intertidal to subtidal.
Table 18.3. Seagrasses recorded in Indonesian waters with brief ecological notes.
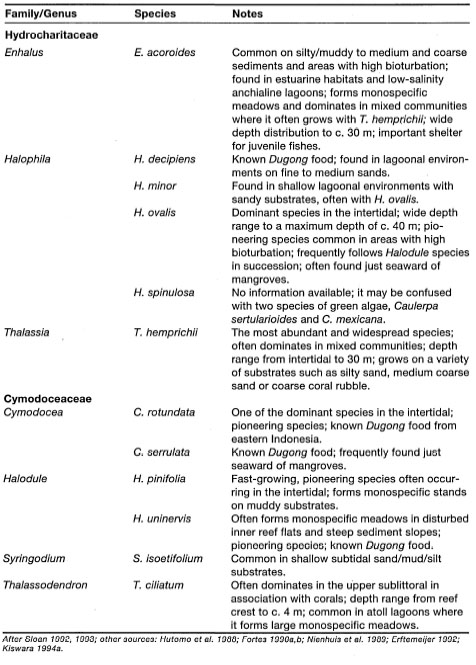
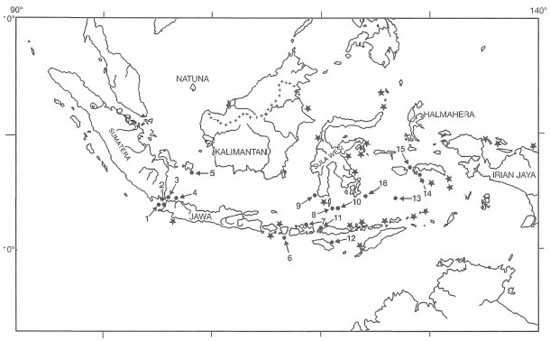
Figure 18.10. Location of all seagrass ecosystem study sites in the Indonesian Archipelago. 1) Sebuku, Legundi Islands (Lampung Bay); 2) Miskam Bay, Sunda Strait; 3) Banten Bay; 4) Jakarta Bay; 5) Belitung Island; 6) Lombok Island; 7) Sumbawa Island; 8) Selayar Island; 9) Spermonde Archipelago; 10) Taka Bone Rate Atoll; 11) Komodo Island; 12) Sumba Island; 13) Lucipara Islands; 14) Nang Bay, Ambon; 15) Seram Island; 16) Kaledupa Atoll, Tukang Besi Islands. Stars indicate locations where seagrass beds were observed by T. Tomascik and A. Mah.
After Kiswara 1994a.
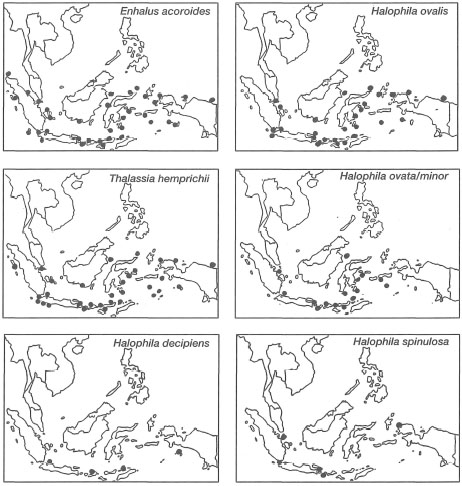
Figure 18.11. Preliminary distribution maps of 12 seagrass species found in the Indonesian Archipelago.
Modified from Kiswara and Hutomo 1985, with additional new information from this work.
Seagrass Community Structure
Seagrasses in the Indonesian Archipelago occur either as monospecific or mixed communities. In monospecific seagrass meadows any one of the 12 known species can dominate the community, which may be a small patch (i.e., < 1 m2) on an intertidal reef flat or a dense and extensive subtidal meadow (i.e., > 100 m2). According to Brouns and Heijs (1990), monospecific seagrass beds are rare in the western tropical Pacific, and are to be considered as an intermediate phase leading towards a more stable mixed community. However, in many sheltered and muddy environments in the Indonesian Archipelago, monospecific stands of Enhalus acoroidesare the climax community, since the environmental conditions are not suitable for other species. An excellent example can be found in an anchialine seawater lake on Maratua Island, East Kalimantan, where Enhalus acoroides forms dense monospecific meadows. No other seagrass species were found in the tidally flushed anchialine lake, and thus the monospecific meadows of E. acoroides are considered as climax communities. Maratua is not an isolated case, since the lagoons of a number of atolls in the Banggai Islands support extensive (> 10 km2) monospecific beds of Thalassodendron ciliatum. Other seagrasses that are frequently found to form monospecific meadows are Thalassia hemprichii, Halophila ovalis, H. uninervis, and Cymodocea serrulata (Nienhuis et al. 1989). Muddy substrates on the seaward edge of mangroves often have monospecific meadows of Halophila uninervis with high biomass.
Species Overview
Enhalus acoroides. Enhalus acoroides is the largest seagrass species and is widespread throughout the archipelago (fig. 18.12). It has been found in a number of environments, ranging from intertidal reef flats to subtidal clearings deep in mangrove forests (e.g., Samama Island). Compared to other seagrasses, E. acoroideshas a rather narrow depth distribution, from intertidal to about 6 m (Brouns and Heijs 1990). In the Spermonde Archipelago it is not found below a depth of c. 5 m (Verheij and Erftemeijer 1993)..It is one of the most common species in silty to muddy sediments, but it roots in medium- to coarse-grained alluvial sediments as well as in coarse-grained carbonate sediments. Because of its long and tough roots (> 50 cm) that provide deep anchorage, E. acoroides often forms monospecific meadows in deeper (2-3 m) lagoonal environments where bioturbation by benthic macroinvertebrates is intense (fig. 18.13).
E. acoroides shows considerable phenotypic plasticity that seems to be related to nutrient availability and environmental conditions (Verheij and Erftemeijer 1993). Two morphotypes of E. acoroides can be recognized. In subtidal habitats, especially in sheltered environments with high nutrient availability, the species is characterized by long (up to 2.5 m; Maratua Island) wide blades and anchored by thick fibrous roots. In sheltered environments with stable sediments, more energy is allocated into growth, resulting in taller plants. In contrast, E. acoroides found in back-reef intertidal reef flats or fringing reef moats are frequently short (30-50 cm) plants with narrow blades. The length of blades seems to be depth-related, since individuals in deeper moats have longer blades. The top of the blades are frequently eroded due to wave energy and exposure to tides (Verheij and Erftemeijer 1993). Fortes (1990) suggested that in high-energy environments more energy is channeled into root/rhizome growth to consolidate the sediments and maintain anchorage.
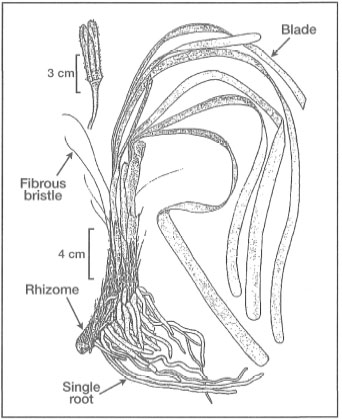
Figure 18.12. External morphological features of Enhalus acoroides.
Modified from Fortes 1990b.

Figure 18.13. Enhalus acoroides often inhabits lagoonal sediments where bioturbation by macroinvertebrates is intense. Note the large burrow mounds.
Photo by Tomas and Anmarie Tomascik.
Halophila spp. Four species of genus Halophila are found in the archipelago. Halophila decipiens (fig. 18.14) and H. spinulosa (fig. 18.15) have a restricted distribution, while H. ovalis (fig. 18.16) and H. ovata (minor) (fig. 18.17) are widely distributed throughout the archipelago. H. spinulosa and H. decipiens often form monospecific meadows in subtidal environments. H. spinulosa has been recorded from Riau, the Sunda Strait, the east Java Sea, Lombok and Irian Java, while H. decipiens has been recorded from locations in Jakarta Bay to the Flores Sea. The location of collection sites suggests that they may be widely distributed, but are rare components of seagrass communities, due to their stricter environmental requirements. With more field work their distribution should expand considerably. Both species prefer clear waters and have a wide depth distribution, ranging from the intertidal to a depth of 40-50 m (Brouns and Heijs 1991). Unlike H. ovalis and H. ovata, which are both dioecious species (i.e., flowers develop on separate individuals), H. decipiens is monoecious, with both male and female flowers developing on the same plant (Verheij and Erftemeijer 1993).
Halophila ovalis has a wide vertical range and occurs from the intertidal zone down to a depth of about 20 m. It is a pioneering species often found growing on recently disturbed sediments, such as mounds of burrowing invertebrates. In the middle eulittoral it is often associated with the smaller, more delicate, H. ovata, which, however, is frequently buried under the sediment and difficult to observe.
Thalassia hemprichii. Thalassia hemprichii is the most abundant seagrass species in the archipelago (fig. 18.18). It is widely distributed and found in a variety of habitats and substrate types. It has a narrow depth distribution, ranging from the lower eulittoral to a depth of 4-5 m. It is frequently the most abundant species of high-energy intertidal reef flats with sandy to coarse rubble substrates. Along the south coast of Bukit Badung Peninsula (south Bali), T. hemprichii often forms monospecific meadows at the seaward margin of the intertidal reef flats where it is subjected to waves and high-velocity tidal currents that exceed 2 m.sec-1. In these environments the blades are only 5-7 cm long, but the root/rhizome network is extensive. The roots and rhizomes form extensive networks (15-20 cm deep) through the sediments, thus not only stabilizing and consolidating otherwise loose sediments, but also providing a strong anchorage that enables the plants to occupy high-energy habitats. Frequently associated with T. hemprichii are calcareous macroalgae such as Halimeda spp., Avrainvillea spp. and Codium spp. Monospecific Thalassia beds are often considered as permanent variants of a mix community in disturbed (i.e., stressful) environments.
Cymodocea spp. This genus has a wide distribution throughout the Indo-Pacific. Two species occur in the Indonesian Archipelago, namely Cymodocea rotundata(fig. 18.19) and C. serrulata (fig. 18.20). The two species are easily distinguishable by their shoot and leaf-blade morphologies (fig. 18.21). Cymodocea rotundata seems to be more common than C. serrulata, and often forms extensive monospecific meadows in the lower eulittoral. It is highly tolerant to subaerial exposure, and is one of the most common seagrasses associated with the intertidal reef flats of fringing reefs, barrier reefs and atolls. C. rotundata is found in a number of reef habitats, but it is most abundant in shallow-water moats of wide fringing reefs. For example, it is one of the dominant seagrasses in the shallow back-reef moat of the extensive Sanur Reef along the southeast coast of Bali. It colonizes a variety of sediments ranging from fine to coarse sands as well as rubble areas where it stabilizes and consolidates the substrate (fig. 18.22).
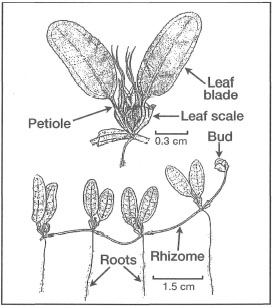
Figure 18.14. External morphological features of Halophila decipiens.
Modified from Fortes 1990b.
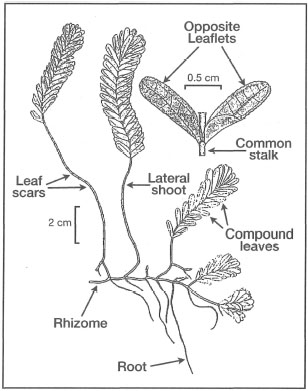
Figure 18.15. External morphological features of Halophila spinulosa.
Modified from Fortes 1990b.
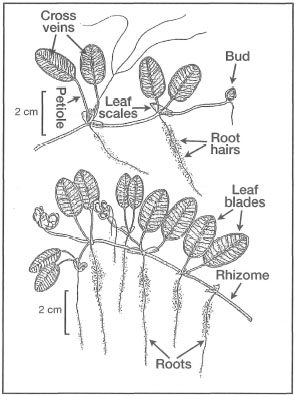
Figure 18.16. External morphological features of Halophila ovalis.
Modified from Fortes 1990b.
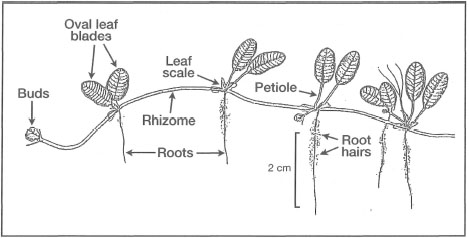
Figure 18.17. External morphological features of Halophila ovata.
Modified from Fortes 1990b.
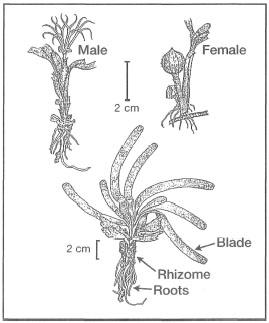
Figure 18.18. External morphological features of Thalassia hemprichii.
Modified from Fortes 1990b.
Cymodocea serrulata is found mainly in subtidal environments to a depth of 3-6m. It is able to grow on a variety of substrates, ranging from silly mud to coarse coral rubble. In relatively sheltered environments with substrates consisting of medium-grained coral sands it was found to form extensive and dense monospecific meadows. In lagoonal mixed seagrass communities, C. serrulata may be the dominant species (fig. 18.23). In Papua New Guinea, highest densities of C. serrulata are usually found on mud-covered coral rubble (Brouns and Heijs 1991).
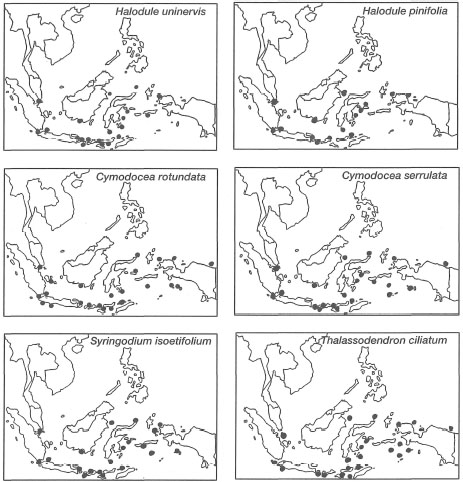
Halodule spp. Both species of Halodule are found in the archipelagic seas. H. uninervis (fig. 18.24) and H. pinifolia (fig. 18.25) have similar distributions and both are widespread throughout the archipelago. H. uninervis tends to be locally more abundant, but extensive monospecific beds of H. pinifolia are not uncommon, and occur mainly on muddy or fine-grained calcareous sands (e.g., Tanjung Reungit in Miskam Bay; Lembata Island) (Kiswara and Tomascik 1994). H. uninervis has a wider depth range (lower eulittoral to 8-10 m) than H. pinifolia, which is usually found in a narrow zone between the middle eulittoral to subtidal (1-2 m). H.uninervis exhibits phenotypic plasticity that seems to be related to depth and degree of exposure. In deeper water the leaves of H. uninervis are wider than in the upper intertidal (Fortes 1990a; Brouns and Heijs 1991).
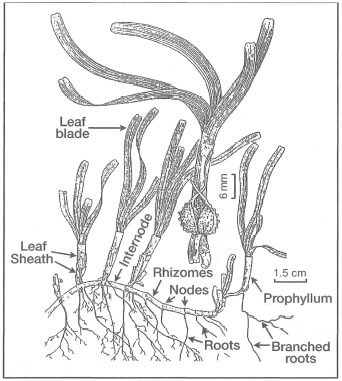
Figure 18.19. External morphological features of Cymodocea rotundata.
Modified from Fortes 1990b.
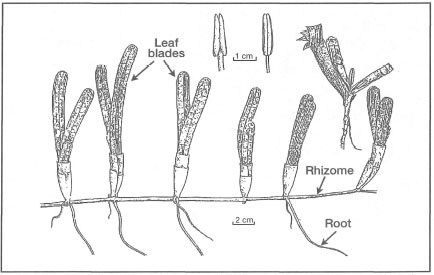
Figure 18.20. External morphological features of Cymodocea serrulata.
Modified from Fortes 1990b.
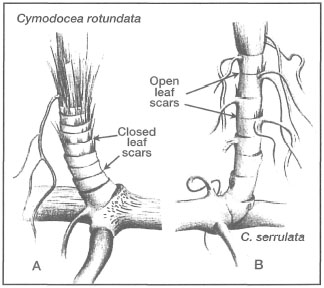
Figure 18.21. The two species of Cymodocea are easily distinguished from each other by the type of leaf scars present on shoots. A) C. rotundata with closed leaf scars. B) C. serrulata with open leaf scar.
From Lanyon 1986.

Figure 18.22. Monospecific meadow of Cymodocea rotundata in the extensive shallow moat of the Sanur fringing reef, southeast coast of Bali. Roots and rhizomes form extensive mats that stabilize and consolidate the substrate. Note gas bubbles on leaf blades.
Photo by Tomas and Anmarie Tomascik.

Figure 18.23. Mixed meadow of Cymodocea serrulata (dominating), C. rotundata and Thalassia hemprichii in the extensive shallow moat of the Sanur fringing reef, southeast coast of Bali.
Photo by Tomas and Anmarie Tomascik.
H.uninervis was observed to form monospecific meadows on exposed inner reef flats as well as on steep sediment slopes consisting of silty to coarse-grained sand. Brouns and Heijs (1991) considered Halodule uninervis as a typical pioneering species able to rapidly colonize newly available substrates.
Syringodium isoetifolium. Syringodium isoetifolium has a wide distribution throughout the archipelago. Its long, thin, cylindrical, hair-like blades easily distinguish this species from all the other seagrasses found in the archipelago (fig. 18.26). It is basically a subtidal species, being very sensitive to exposure and desiccation. The sensitivity to desiccation is most likely related to its leaf morphology. Most seagrasses are tolerant of desiccation for relatively long periods, because their broad leaves overlap one another, thus creating areas where water is trapped and high relative humidity is maintained. The cylindrical leaves of S. isoetifolium do not offer this type of protection, and therefore S. isoetifolium is seldom found above the lower low water, large tide mark (LLWLT).
S. isoetifolium has a high nutritional value and seems to be the preferred seagrass for Dugong dugon. A dugong kept at the Surabaya Zoo is fed mostly S. isoetifolium(35 kg.day-1).
Thalassodendron ciliatum. Not too long ago it was believed that Thalassodendron ciliatum had a rather restricted distribution in the archipelago (Nienhuis 1989). It is relatively rare in the western parts of the archipelago, but very common in the eastern parts (Brouns and Heijs 1991; T. Tomascik and A. Mah, pers. obs.).
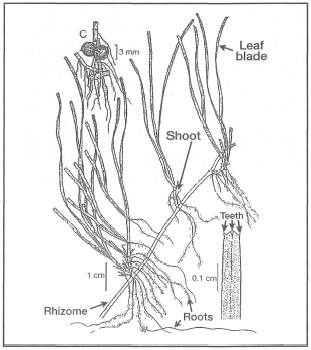
Figure 18.24. External morphological features of Halodule uninervis.
Modified from Fortes 1990b.
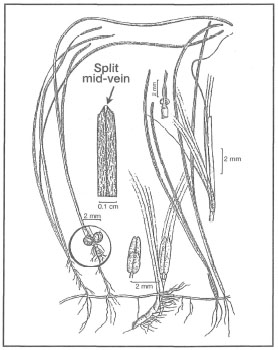
Figure 18.25. External morphological features of Halodule pinifolia.
Modified from Fortes 1990b.
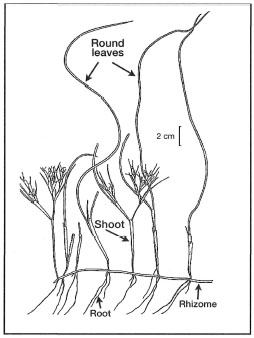
Figure 18.26. External morphological features of Syringodium isoetifolium.
Modified from Fortes 1990b.
T. ciliatum was recently found in the Riau Islands, northwest Java Sea (Kiswara, pers. comm.). The species generally occurs in stable sublittoral environments, most frequently at the seaward margin of reefs (i.e., fringing reefs to atolls). With its strong woody rhizomes and roots, it is able to root in a variety of sediment types, including coarse coral rubble. However, it has also been observed on solid reef substrates. The fringing reef along the Nusa Dua coast (south Bali) has a well-developed spur-and-groove zone facing the Indian Ocean. The u-shaped grooves are 1 to 1.5 m deep, running almost perpendicular to the incoming swell. The inner walls of the grooves are heavily overgrown by T. ciliatum which has been able to root on solid highly exposed reef substrate. The shoots are 10-15 cm long and extremely difficult to remove.
T. ciliatum has a relatively unique morphology, with long ribbon-like shoots and highly lignified woody rhizomes and roots (fig. 18.27). According to den Hartog (1970), the branching of the erect stems in T. ciliatum is unusual for seagrasses. Based on our observations it seems that branching frequency may be related to wave exposure and depth. Brouns (1985) reported that branching in T. ciliatum was very common in the Flores Sea (i.e., Keladupa Atoll and Taka Bone Rate Atoll). The study areas were two large atolls with extensive T. ciliatum meadows on the seaward margins of the reefs, thus most likely high-energy environments. A brief survey of extensive T. ciliatum meadows along the seaward margin of a large barrier reef surrounding Sago Island, Banggai Islands, revealed that branching was also very common. The branched erect stems (two maximum) were c. 10-15 cm long, and the seagrass covered the reef crest like a carpet. The south- and southwest-facing reef crest is exposed to the Southeast Monsoon, however, the presence of fine-branching Acropora spp. suggests that the reef is not subjected to excessively strong wave action. However, high-velocity tidal currents were observed. In contrast to the relatively short branched stems observed at the Banggai Islands, the stems of T.ciliatum found in mixed lagoonal seagrass beds at the Salabanka Islands were long (50-75 cm) and unbranched (fig. 18.28). It appears that in all high-energy environments (e.g., south Bali, south Lembata Island, Panaitan Island, Banggai Islands), T. ciliatum has shorter branched stems than in sheltered lagoonal environments (i.e., calm, turbid), where it occurs in mixed communities. Additional research is needed to determine whether this relationship has ecological implications.
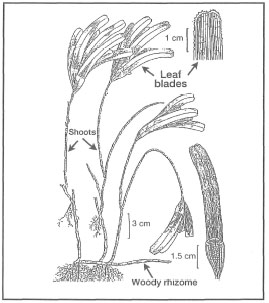
Figure 18.27. External morphological features of Thalassodendron ciliatum.
Modified from Fortes 1990b.
Mixed Seagrass Communities
Seagrass Associations of 2-3 Species. Throughout the Indonesian Archipelago multi-species or mixed seagrass meadows consisting of up to eight species are a relatively common occurrence (Nienhuis et al. 1989). This is one of the key structural features that clearly distinguishes Indonesian seagrass communities from those of the Caribbean, where monospecific meadows are most common. Multi-species associations may be divided into those with 2-3 species and those with more than four species (Nienhuis et al. 1989; Brouns 1991). Seagrass meadows dominated by a particular 2-3 species association are among the most common seagrass communities in the archipelago. A number of 2-3 species associations can be recognized. Among the most common intertidal reef flat associations is that of Thalassia hemprichii and Cymodocea rotundata (Brouns and Heijs 1991). This association occurs on a variety of substrates, but does not necessarily occur in all seagrass habitats. For example, while it is not a distinct association in the extensive seagrass beds on the south coast of Lombok (Kiswara and Winardi 1994), it is a dominant association on the intertidal reef flats along the south coast of Bali, as well as the seaward margins of the reef flats on the west coast of Lembata Island, and the back-reef environments of the south coast (fig. 18.29).

Figure 18.28. Mixed seagrass bed in Salabanka Islands. Thalassodendron ciliatum found in a mixed sea-grass bed with Enhalus acoroides, Cymodocea serrulata, Thalassia hemprichii and Halophila oval is.Note the long unbranched stems of T.ciliatum.
Photo by Tomas and Anmarie Tomascik.
The most common seagrass association in the archipelago is that of Enhalus acoroides and Thalassia hemprichii. Brouns and Heijs (1991) mention Halophila ovalisas an occasional member of this association in seagrass meadows in Papua New Guinea. In the Indonesian Archipelago, however, Halophila ovalis occurs with a number of different seagrass species, and as a result it is difficult to place it with any one particular association. Nonetheless, it was found to be associated with Cymodocea rotundata on the high-energy reef flats at Nusa Dua, on the south coast of Bali, where the surface sediments consisted of 60%-70% foraminiferan tests (i.e., Baculogypsina sphaerulata) (fig. 18.30).
The E. acoroides -T. hemprichii association is usually found in relatively sheltered environments. It was found to be the dominant association on the reefs of Kepulauan Seribu (Azkab 1991), where it has been documented on 75% of reefs that were studied. It has been reported that the relative abundance of each species varies depending on location (Brouns and Heijs 1991). These differences seem to be associated with the degree of wave exposure. T. hemprichii frequently dominates, in terms of abundance, in areas exposed to waves, while communities in more sheltered environments are numerically dominated by E. acoroides. The association is not a distinct zone on high-energy reefs (exposed to Indian Ocean swell) of south Lombok, even though both species occur in the area (Kiswara and Winardi 1994).
Brouns and Heijs (1991) described a number of 2-3 species associations common in the waters of Papua New Guinea. Their Halodule uninervis and Halophila ovalis association was observed on a gentle seaward sandy slope of a small patch reef in the Salabanka Islands (East Kalimantan). This seagrass association was found on a rippled (fast-flowing currents are common) medium-grained coral sand substrate in a wide band between 5-8 m depth and extended for 200-300 m along the slope. It is also common in calmer lagoonal environments where it often covers large burrow mounds (Brouns and Heijs 1991). On the south and east coasts of Bali (Nusa Dua and Sanur, respectively) the association is joined by Cymodocea rotundata in the upper sublittoral zones (i.e., moat), where the sediments are dominated by highly mobile foraminiferan (mainly Baculogypsina sphaerulata) tests (i.e., foraminiferal sand). In general, the presence of if. ovalis and H. uninervis, which are considered as pioneer species, is indicative of relatively unstable substrates (Brouns and Heijs 1991). Their ability to colonize highly mobile intertidal and subtidal substrates is due to their relatively rapid vegetative propagation, rapid shoot turnover and high tolerance to desiccation and exposure.
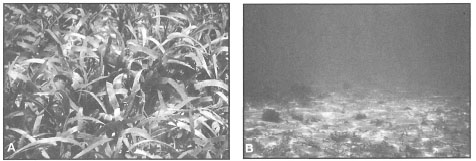
Figure 18.29. Seagrass meadow consisting of Cymodocea rotundata and Thalassia hemprichii association. A) Dense meadow in the Sanur Reef lagoon, Bali. Note the file snake Acrochordus granulatus at centre. Sanur, Bali. B) Sparse meadow on the west coast of Lembata Island, East Nusa Tenggara.
Photos by Tomas and Anmarie Tomascik.

Figure 18.30. Seagrass meadow consisting of Cymodocea rotundata, Thalassia hemprichii and Halophiia ovalis association.
Photo by Tomas and Anmarie Tomascik.
In the Flores Sea the intertidal zone is characterized by pioneer species, frequently dominated by Halophiia ovalis, Cymodocea rotundata and Halodule pinifolia (Nienhuis et al. 1989). Thalassodendron ciliatum may dominate in the upper sublittoral (1-3 m depth below LW) zone, where it is able to grow on a variety of substrates ranging from medium- to coarse-grain sand and coral rubble to silty sand.
Mixed Associations with 4-8 Species. Mixed seagrass associations are those with more than three seagrass species. Mixed meadows have been reported to be abundant in sheltered environments characterized by sandy (not muddy), stable and nearly-horizontal sediments (Hutomo et al. 1988). However, in sheltered habitats, high bioturbation due to burrowing activities of shrimps and other macroinvertebrates tends to decrease seagrass diversity and density, and favour pioneering species such as Halophila ovalis and Halodule uninervis (Hutomo et al. 1988). In general, mixed seagrass associations are not found in: 1) "extremely" sheltered low-energy environments where sediments are dominated by fine-grained sand to silty muds (e.g., Komodo); 2) on recently deposited sediments (e.g., Taka Bone Rate Atoll); 3) on steep unstable sediment slopes (e.g., Sumbawa); and 4) in upper intertidal where subaerial exposure during low tides causes severe desiccation (Nienhuis et al. 1989).
The most diverse seagrass associations are found in reefal habitats in the upper sublittoral zone. For example, Nienhuis et al. (1989) described an eight-species association from various reefal habitats in the Flores Sea (Selayar Island, Taka Bone Rate Atoll, Komodo and Sumbawa) (fig. 18.31). In decreasing order of frequency of occurrence the eight-species association was comprised of Thalassia hemprichii (occurs in 96% of samples), Syringodium isoetifolium (83%), Cymodocea rotundata (83%), Enhalus acoroides (65%), Halodule uninervis (65%), Cymodocea serrulata (61%), Halophila ovalis (35%) and Halodule pinifolia (9%) (Nienhuis et al. 1989). In many habitats, each of the species can be locally very abundant and dominate the community. The association is found in highly stable environments, which most frequently are slightly inclining or almost horizontal sand- and rubble-covered reef flats (fig. 18.32) (Nienhuis et al. 1989). Above-ground biomass is very high and the extensive root/rhizome networks bind and consolidate reef flat sediments.
In a recent multidisciplinary study on the south coast of Lombok, Kiswara and Winardi (1994) recorded 11 seagrass species from two locations (i.e., Kuta and Gerupuk Bays) (fig. 18.33). With 11 species, Lombok has the highest diversity of any single island in the archipelago. Note that it is one of the few islands to be intensively studied. Highest diversity was observed at the more sheltered sites at Gerupuk Bay, which is protected from both the southwest and southeast swells. Higher-energy conditions in Kuta Bay are reflected in lower percentage cover of Enhalus acoroides (35%) than in the sheltered Gerupuk Bay (50%). However, lower diversity in Kuta Bay may also be a result of higher water turbidity associated with land runoff from a large river (Kiswara and Winardi 1994). Collection of Halophila spinulosa in Gerupuk Bay, and Thalassodendron ciliatum in both Kuta and Gerupuk Bays, are new records of these species in this area. Note, however, that both species were observed along the south coast of Bali (T. Tomascik, pers. obs.). In both bays seagrasses occur in monospecific meadows, in 2-3 species associations as well as in mixed associations of up to six species (fig. 18.34).
Seagrass-Associated Flora and Fauna
Benthic Macrophytes. Seagrasses are associated with a large variety of macroalgae. Kiswara (1991a), for example, reported that Gracillaria lichenoides, which is an economically important species, is one of the dominant macrophytes in seagrass meadows near Lontar, West Java. Atmadja (1992) reported that fishermen at Benoa, Bali, and along the coast of West Lombok collect seven species of seaweeds (i.e., Eucheuma arnoldi, E. spinosum, Gelidiella acerosa, Gelidiopsis intricata, Gracillaria eucheumoides, G. lichenoides and Hypnea cervicornis) from mixed seagrass meadows consisting of Cymodocea serrulata, Halodule uninervis, Thalassia hemprichii and Thalassodendron ciliatum. In many areas (e.g., Salabanka) economically valuable Caulerpa spp. are abundant components of seagrass communities, but remain unused. In the Philippines, seagrass-associated macrophytes are an important economic resource, harvested for the production of agar (e.g., Gracillaria and Gelidiella) as well as animal feed, fertilizer and alginates (e.g., Sargassum spp.) (Fortes 1990a). In Salabanka, Central Sulawesi, seaweed farming in lagoonal environments dominated by mixed seagrass communities is becoming an important economic activity (fig. 18.35).
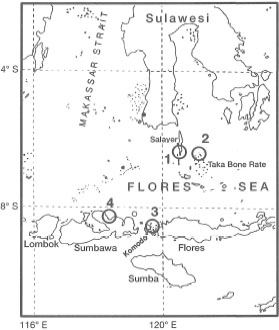
Figure 18.31. Location of seagrass sampling stations during the Snellius-ll Expedition to eastern Indonesia. 1 - Selayer Island; 2 Taka Bone Rate Atoll; 3 - Komodo Island; 4 - Sumbawa Island (Bima and Sanggar Bays).
From Nienhuis et al. 1989.
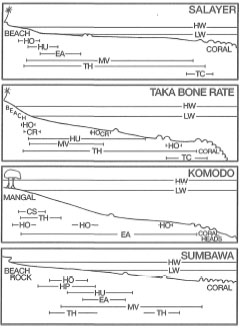
Figure 18.32. General zonation pattern of seagrasses along transects perpendicular to the shoreline from reefal habitats in Flores Sea (see fig. 18.31 for location). TH- Thalassia hemprichii; Sl-Syringodium isoetifolium; CR-Cymodocea rotundata; EA- Enhalus acoroides;HU-Halodule uninervis; CS-Cymodocea serrulata; HO- Halophila ovalis; HP-Halodule pinifolia; MV- mixed vegetation. Tidal range 1-1.5 m. Transect length: Selayar and Taka Bone Rate, 500 m; Komodo and Sumbawa, 300 m.
From Nienhuis et al. 1989.
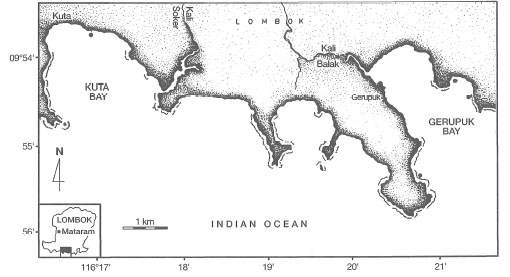
Figure 18.33. Location of Kuta Bay and Gerupuk Bay on the south coast of Lombok Island.
From Kiswara and Winardi 1994.
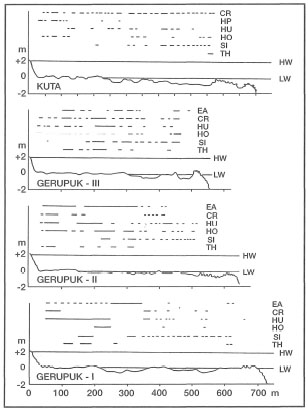
Figure 18.34. Zonation of seagrasses perpendicular to the shoreline at Kuta Bay and Gerupuk Bay study sites.
TH = Thalassia hemprichii;
SI = Syringodium isoeti folium;
CR = Cymodocea rotundata;
EA = Enhalus acoroides;
HU = Halodule uninervis;
HO = Halophila oval is;
HP = Halodule pinifolia.
HL = high water;
LW = low water.
From Kiswara and Winardi 1994.
In a long-term study of seagrass communities conducted in the Spermonde Archipelago, Verheij and Erftemeijer (1993) recorded 117 species of macroalgae associated with seagrass meadows in five different habitats. The macroalgal community comprise 50 species of Chlorophyta, 17 species of Phaeophyta, and 50 species of Rhodophyta (Verheij and Erftemeijer 1993). However, only 13 species were found exclusively in association with seagrasses, namely Avrainvillea stellata, Caulerpa buginense, C. racemosa ecad corynephora, Chaetomorpha crassa, Dictyota ciliolata, D. linearis, Hydroclathrus tenuis, Actinotrichia fragilis, Gracillaria salicornia, Trichogloea requierii, Fosliella sp. Mastophora rosea, and Neogoniolithon brassica-floridum ecad frutescens (Verheij and Erftemeijer 1993). The remaining 104 species are widely distributed throughout the Spermonde Archipelago where they inhabit a variety of reefal and non-reefal habitats (Verheij and Prud'homme van Reine 1993). Species composition of macroalgae associated with seagrass beds is dependent to a large extent on substrate type and degree of exposure. On inshore intertidal terrigenous mud flats, dominated frequently by monospecific stands of Enhalus acoroides and Halodule pinifolia, the most abundant macroalgae were Caulerpa racemosa, Udotea flabellum, Ulva reticulata, Gracillaria salicornia and G. verrucosa (Verheij and Erftemeijer 1993). In areas where terrigenous sediments are mainly medium to coarse sands, and support mixed seagrass associations, the dominant macrophytes are Halimeda opuntia forma opuntia, Dictyota linearis and Amphiroa fragilissima (Verheij and Erftemeijer 1993). Further offshore carbonate sands replace terrigenous sediments and mixed seagrass communities dominate. In these environments the dominant macroalgae include Avrainvillea obscura, Enteromorpha clathrata, Hydroclathrus clathratusi and Hydrolithon reinboldii (Verheij and Erftemeijer 1993). The general pattern that emerged from the study was that macroalgae associated with seagrass beds have their greatest diversity on reef flats of patch reefs and coastal reefs (Verheij and Erftemeijer 1993).
Seagrass Epiphytes. The term seagrass epiphyte refers to all autotrophic (i.e., primary producers) above-ground organisms living permanently attached to rhizomes, shoots and leaves of seagrasses. However, the term has often been used to refer to all organisms (animal or plant) growing on seagrasses (Russell 1990). We prefer to use the term epifauna for all heterotrophic organisms attached to seagrass parts above sediments, while infauna is used to refer to organisms living within the sediments among the seagrass rhizome/root networks. Seagrass blades (leaves) often have the highest abundance of epiphytes, since they offer solid substrates with access to light, nutrients and water exchange. Unlike many marine seaweeds (e.g., Phaeophyta), seagrasses do not possess strong chemical defenses (e.g., phenolic compounds), which allows them to be used as living substrates by a variety of sessile and motile organisms.
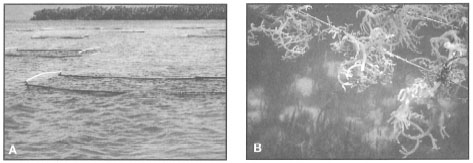
Figure 18.35. Floating raft technique (A) is a profitable seaweed farming enterprise in lagoonal habitats dominated by mixed seagrass communities (B) (Enhalus acoroides, Cymodocea serrulata, Thalassia hemprichii and Thalassodendron ciliatum) in Salabanka, Central Sulawesi.
Photo by Tomas and Anmarie Tomascik.
Based on the general external morphology of seagrasses, Borowitzka and Lethbridge (1989) described the following five species groups that provide distinct environments for the attachment of epiphytes and epifauna:
i) Species with long (5-200 cm) wide (2-18 mm) strap-shaped leaves often forming upper canopy: e.g., Enhalus acoroides, Cymodocea rotundata, C. serrulata, Thalassia hemprichii.
ii) Species with long (10-75 cm) upright woody (lignified) stems, with distichously attached leaves forming dense canopy in monospecific meadows: e.g., Thalassodendron ciliatum.
iii) Species with long (10-35 cm) subulate leaves: e.g., Syringodium isoetifolium.
iv) Species with fine narrow (1-3 mm), sometimes long (10-18 cm), linear leaves: e.g., Halodule pinifolia, H. uninervis.
v) Species with short delicate elliptic, ovate lanceolate or linear leaves often forming understory in mixed associations: e.g., Halophila ovalis, H. ovata, H. spinulosa, H. decipiens.
Detailed seasonal (i.e., long-term) quantitative studies on seagrass epiphytes are not available from Indonesia. However, numerous studies have demonstrated that a significant portion of the total primary production in seagrass meadows is attributed to the epiphytic cyanobacteria and algae (Heijs 1985; Borowitzka et al. 1990; Tomasko and Lapointe 1991; Klumpp et al. 1992; Pollard and Kogure 1993). The high contribution of epiphytes to the total seagrass community primary production is in part due to the fact that the surface area occupied by the epiphytes (i.e., seagrass surfaces) is up to 20 times that of the sediment surface area occupied by the seagrasses (Couchman 1987).
Species richness and production of seagrass epiphytes varies depending on seagrass species as well as on environmental condition and habitat type (e.g., depth). Life span of seagrass leaves is an additional factor in determining the diversity and biomass of epiphytic communities (Borowitzka and Lethbridge 1989), since leaf turnover rates among the seagrass species vary greatly according to the basic metabolic process of each species, as well as due to herbivore grazing intensity. In the Spermonde Archipelago, South Sulawesi, the epiphytic algal community on seagrass leaves consisted of 18 species, while only nine species of epiphytes were found on the stems (Verheij and Erftemeijer 1993). In the Philippines, Enhalus acoroides supports the highest number of epiphytes (Tiquio and Fortes 1994). In monospecific seagrass communities (Cymodocea rotundata, C. serrulata, Syringodium isoetifolium and Halodule uninervis) in Papua New Guinea, epiphytic algae have been shown to contribute as much as 24% to the total above-ground biomass production, and 44% to above-ground primary production (Heijs 1985). The most important epiphytes, out of about 55 species, common to all four seagrass species were the coralline encrusting algae (e.g., Fosliella farinosa, F. lejolisii, Melobesia membranacea), Cyanophyta and Rhodophyta (Ceramium gracillimum, Polysiphonia savatierii, Audouinella spp.) (Heijs 1985). In a similar study in Fiji, Pollard and Kogure (1993) found that the epiphytic component on the shoots of Syringodium isoetifolium accounted for 22%-65% of the total primary production. Epibenthic algae present on the surface layer of seagrass sediments are also an important component of seagrass communities. Pollard and Kogure (1993) have reported that the epibenthic algal community accounts for up to 25% of total seagrass productivity.
In addition to the significant production of organic carbon, the remains of calcareous epiphytes (i.e., crustose coralline algae; Corallinaceae) are an important source of CaCO3. Walker and Woelkerling (1988) estimated that the red coralline algae on the leaves and stems of Amphibolis antarctica (Shark Bay, Western Australia) account for some 200 tonnes.km-2 of CaCO3. Encrusting coralline algae are considered as a pioneering group with rapid development to sexual maturity (Borowitzka and Lethbridge 1989).
The epiphytic and epibenthic communities are integral components of the three-dimensional seagrass environment by providing abundant food resources for numerous invertebrate and vertebrate grazers. Klumpp et al. (1992) point out that in terms of nutritional value, epiphytic communities are far superior to sea-grasses (epiphyte C:N ratios are 9:18; seagrass C:N ratios are 17:30). Thus high biomass of seagrass epiphytes adds considerably to the overall nutritional value of plants. Nonetheless, Birch (1975) compared tropical seagrass meadows to poor pastures. In fact, it has been a popular view that only a few animals graze directly on living seagrasses, among the best known being the dugongs (Dugong dugon) and sea turtles (Chelonia mydas) (Thayer et al. 1984). To dispel the notion, McRoy and Helfferich (1980) compiled a list of 154 species of direct seagrass consumers, and noted that grazing on live seagrasses was more common in the tropics. The earlier view, concerning the low abundance of living seagrass consumers, was attributed to the fact that seagrasses are composed primarily of refractory material, such as cellulose (Mann 1988), and are extremely low in nitrogen (Koike et al. 1987). Both sea turtles and dugongs have evolved specialized digestive tracts to extract as much nutrition from seagrasses as possible (Lipkin 1975; Bjorndal 1980; Garnett et al. 1985). Seagrass consumers also show preference for particular parts of the plant, thus maximizing their nutritional intake (Bjorndal 1980; Ogden 1980). The nutritional importance of the epiphytic seagrass community in the diets of these large animal grazers remains largely unknown (Borowitzka and Lethbridge 1989).
In areas subjected to higher nutrient inputs, epiphytic algae increase substantially in biomass to the detriment of seagrasses. Seagrass epiphytes are in fact very useful indicators of seagrass health. Tomasko and Lapointe (1991) have shown that increased nutrient concentrations in the water column will increase epiphytic biomass and substantially reduce the growth rates of seagrass rhizomes, which results in lower shoot density, and thus, lower overall seagrass primary production (Tomasko and Lapointe 1991). Eutrophication of coastal waters is thus a serious concern to seagrass health, especially in areas such as Sanur, Bali, where large hotels are discharging nutrient-rich effluents directly onto the reef. While low nutrient subsidy may enhance and maintain high seagrass primary production rates without detrimental effect to the community as a whole, experimental research to determine water-quality guidelines is sorely lacking.
Fauna. Seagrass communities harbour a wide range of benthic, demersal and pelagic organisms that are either permanent residents of the system or transients. The transient species are frequently juvenile stages of numerous organisms that seek food and shelter during critical parts of their life cycles, or they may be daily visitors that use seagrass beds as feeding grounds. Many of the permanent or transient epibenthic species are of significant economic value, shrimps and prawns being the most important. For the sake of clarity, and not necessarily for any particular ecological or biological reasons, four major faunal groups are recognized, namely 1) Infauna (i.e., living within the sediments); 2) Motile epifauna (i.e., motile fauna associated with the surface sediment layer); 3) Sessile epifauna (i.e., attached organisms to any part of a seagrass); and 4) Epibenthic fauna (i.e., large mobile fauna within seagrass beds) (Howard et al. 1989).
Seagrass-associated fauna remains one of the most open and exciting fields of research for Indonesian scientists. With a minimum of 30,000 km2 of seagrass beds spread throughout the archipelago, from Pulau Weh in Aceh to the coastal waters of Marauke, Irian Java, the potential for research is immense. It is, however, essential that a research protocol is established to move away from the 'haphazard' approach that dominates current research programs. The new and exciting multidisiplinary studies on seagrass ecosystems along the south coast of Lombok are a move in the right direction. Recent studies on seagrass-associated fauna have focused on establishing species lists, diversity patterns, obtaining basic information on ecological parameters such as abundance and biomass of various seagrass-associated taxa, and trophodynamics. With few exceptions (Suhartati 1994; Susetionto 1994), the majority of seagrass-associated faunal studies (see table 18.4 for classification) dealt with infaunal macrofauna, motile epifauna and epibenthic fauna (Aswandy and Hutomo 1988; Kiswara et al. 1991,1992; Aswandy and Kiswara 1992a; Mudjiono et al. 1992; Aziz 1994; Aziz and Sugiarto 1994; Hutomo and Parino 1994; Moosa and Aswandy 1994; Mudjiono and Sudjoko 1994; Pristiwady 1994).
Meiofauna. Susetiono (1994) reported on meiofauna associated with monospecific Enhalus acoroides seagrass beds on the south coast of Lombok. The sediment infauna consisted of nematodes, foraminiferans, copepods, ostracods, turbelarians and polychaetes. The high abundance of nematodes (i.e., nematodexopepod abundance ratio index) was indicative of nutrient enrichment which is most likely associated with land runoff. Actively emerging meiofauna observed were copepods, nematodes, amphipods, cumaceans and ostracods. Generic- or species-level analyses have not been conducted thus far. Based on available information from Kuta Bay, Susetiono (1994) constructed a simplified food web within the Enhalus acoroides sea-grass beds (fig. 18.36).
Benthic foraminifera are an important component of seagrass communities, but have received only rudimentary attention (Suhartati 1994). In the Kepulauan Seribu patch reef complex, seagrass beds are abundant and frequently dominated by Enhalus acoroides and Thalassia hemprichii association (Azkab 1991). Benthic foraminifera in these two species associations are dominated by the Suborders Miliolina and Rotaliina (Suhartati 1994). The miliolinids are characterized by smooth, porcelaneous tests consisting of calcite crystals, while the rotaliinids have glassy, double-walled tests consisting of radial laminated hyaline calcite. The most abundant rotaliinids were Ammonia beccarii, A. umbonata, Calcarina calcar, Elphidium advenum, E. crispum, E. craticulatum and Rosalina bradyi. Genus Ammonia is a well-known euryhaline group, common in shallow-water tropical environments. The presence of Calcarina calcar (Family Calcarinidae) is indicative of coral reef habitats, which is not surprising, since the seagrass beds studied were in reefal habitats. The abundance of Elphidium spp. (Family Elphidiidae) is interesting, since this euryhaline, shallow-water species is extremely tolerant of low salinities, and can be found long distances up into estuaries (Lee et al. 1985). The miliolinids are represented by Adolesina semistriata, Miliolinella sublineata, Quinqueloculina granulocostata, Q. parkery, Q. sp., Spiroloculina communis, Spirolina cilindricea and Triloculina. tricarinata. Both genera, Quinqueloculina and Triloculina (Family Miliolidae), are characteristic of shallow tropical waters.
Table 18.4. Major categories of taxa in the main faunal groups.
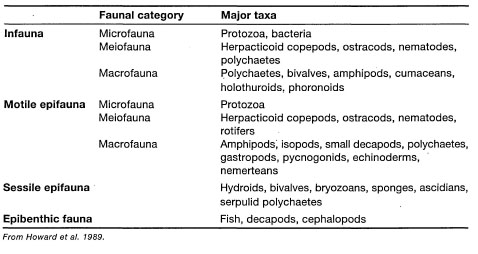
Figure 18.36. Simplified food web for monospecific meadows of Enhalus acoroides seagrass.
From Susetiono 1994.
Crustaceans. Seagrass-associated Crustacea are key components of seagrass food webs. The infaunal and epifaunal Crustacea form an important link between the primary producers and higher trophic levels, since during their juvenile and adult lives they are a major food source for a variety of seagrass-associated fish and invertebrates (fig. 18.37). Recent gut analyses studies of seagrass-associated fish fauna, on the south coast of Lombok (Peristiwadi 1994), demonstrated that crustaceans are the dominant food source (fig. 18.38). However, Kikuchi (1980) pointed out that, in temperate seagrass ecosystems, invertebrate consumption of living seagrass was rather rare, and that detrivory may dominate the food habits of the majority of seagrass-associated crustaceans (fig. 18.39). In many temperate areas crabs and isopods are the dominant crustacean grazers. Nienhuis and van Ierland (1978) found that isopods were responsible for over 75% of plant consumption in European seagrass beds. Thus far, similar studies from Indonesia are sorely lacking. The role of gammarid amphipods in the seagrass ecosystem food web has not been fully assessed. There are some doubts as to whether amphipods feed on live seagrass under natural conditions, even though their ability to do so was demonstrated in laboratory experiments (Kirkman 1978). The abundance of epiphytes with their superior nutritional value offers crustaceans an alternate nutritional pool to choose from.
Aswandy and Hutomo (1988) recorded 28 species of crustaceans in Ban ten Bay seagrass beds. Two species of amphipods, namely Apseudeus chilkensis and Eriopisa elongata, were the most abundant crustaceans in Enhalus acoroides meadows in Grenyang Bay. Until recently little quantitative work has been conducted on sea grass-associated crustacean fauna. Moosa and Aswahdy (1994) have compiled a comprehensive species list for seagrass meadows in Kuta and Gerupuk Bays on the south coast of Lombok. A list of 70 crustacean species was compiled from both bays, however, many specimens were apparently collected from coral rubble areas adjacent to the seagrass meadows, and thus whether they are in fact seagrass-associated remains doubtful. Of the 70 species of crustaceans, only one hipollitid shrimp, Tozeuma sp., has special morphological adaptations to live specifically in seagrass meadows. A temperate member of this genus, Tozeuma carolinense (Caridea), is a common inhabitant in eel grass beds along the east coast of United States, and is green in colour (Barnes 1980). Stomatopods were also found in the seagrass beds. This relatively diverse group of predators has a wide distribution, but it seems that only Pseudosquilla ciliata is an obligate seagrass-associated species (Moosa and Aswandy 1994). However, stomatopods are common on adjacent intertidal reef flats. Among the most colourful of all reef-associated stomatopods is Odontodactylus scyllarus (Squillidae), which is an active mollusc predator (fig. 18.40). This voracious predator has a wide distribution and lives in a number of reef habitats, preferring crevices. However, on reefs where seagrasses are growing along the seaward edge of the reef flats, O. scyllarus was observed to forage into the outer periphery of these habitats, apparently in search of molluscs which are abundant in seagrass beds (T. Tomascik, pers. obs.; Bunaken 1993).
Predatory crustaceans associated with seagrass beds are often common under natural conditions (i.e., unexploited by local inhabitants). Among the most common are the box crabs, Family Calappidae. The box crabs are usually burrowed in sand just below the surface, and are active gastropod predators (fig. 18.41). Their right claw is specialized for crushing gastropod shells. The crushing claw has a large tubercle at the base of the movable finger that assists in piece-by-piece crushing of gastropod shells. Box crabs are common throughout the archipelago and three species were recorded in south Lombok seagrass meadows (i.e., Calappa hepatica, C. calappa and Matuta banksii). The swimming crabs of Family Portunidae are frequently abundant in seagrass meadows, and were recorded in Lombok (Moosa and Aswandy 1994). Seagrass beds are also critical habitats for the juvenile stages of Portunus pelagicus, an economically valuable species. In this family (Portunidae), the fifth pair of legs is adapted for swimming, which is unique since most crabs cannot swim. The specially adapted legs act as "propellers". These crabs spend most of their time buried just beneath the surface where they wait to ambush any passing fish or invertebrates; it is a highly aggressive species. However, many species of fish prey on it as well. The males are larger than females. The portunid crabs have a unique mating arrangement, which they also share with the Cancridae. During mating, the male carries the female beneath him, with her carapace beneath his sternum. All movements, walking and swimming, are done in unison. When the female is ready to molt the male releases her, and copulation occurs shortly after (fig. 18.42).
Seagrass meadows are recognized critical habitats for the commercially important penaeid prawns (i.e., Penaeus esculentus and P. semisulcatus) (Bell and Pollard 1989; Coles et al. 1993; Mellors and Marsh 1993; Watson et al. 1993) and spiny lobster (Panulirus ornatus) (fig. 18.43) (Bell and Pollard 1989; Poiner et al. 1989), which depend on seagrass beds for food as well as shelter during the postlarval and juvenile stages of their life cycles. In experimental laboratory studies, Hill and Wassenberg (1993) found that Penaeus esculentus spent about 80% of their non-swimming time in seagrass. During daylight hours P. esculentus adults (i.e., large individuals, 15 mm CL) were found buried in the substrate, whereas smaller individuals were found buried, standing on the bottom or clinging to the seagrasses. Similar results were obtained for P. semisulcatus, thus supporting numerous field observations.
Figure 18.37. Crustacean larvae are a major food source for fish and other organisms. A) Decapod larva. B) Zoea larva.
Photos courtesy of Ft. Steene, Cairns.
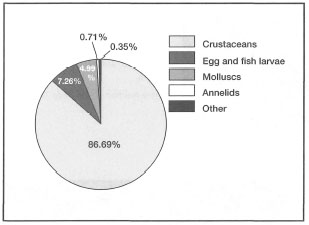
Figure 18.38. Results of gut analyses of various seagrass associated fauna revealed that in the seagrass ecosystems along the south coast of Lombok crustaceans are the dominant fish food.
Source: Peristiwady 1994.

Figure 18.39. Amphipods are a relatively unknown nutritional component of seagrass beds, yet they are a major link between the primary producers and consumers in higher trophic levels.
Photo courtesy of R. Steene, Cairns.

Figure 18.40. Odontodactylus scyllarus is a predatory mantis shrimp (Squillidae) that actively preys on molluscs. It has powerful claws capable of crushing mollusc shells.
Photo courtesy of R. Steene, Cairns.

Figure 18.41. Predatory crabs such as Calappa sp. hunt for gastropods and other molluscs. They are able to crush the molluscan shells with their powerful specialized right claws. They feed mainly at night and remain buried just beneath the sediment surface during daylight hours.
Photo courtesy of R. Steene, Cairns.

Figure 18.42. Portunus pelagicus mating pair. Note the female beneath the male.
Photo courtesy of R. Steene, Cairns.
The association of a number of penaeid prawn species with seagrass beds during certain periods of their life cycles is well established (fig. 18.44). The question that still remains relatively unanswered, is which component(s) of the seagrass ecosystem contribute most to the penaeid energy requirements. Do the penaeids use seagrass habitats for protection or food, or both? Wassenberg and Hill (1987) reported that gut contents of Penaeus escukntus collected from seagrass meadows in Moreton Bay, Australia, contained seeds of Zostera capricornis, the dominant seagrass. On the other hand, Kitting et al. (1984), using the 13C/12C stable isotope ratios, were able to determine that P. aztecus and P. duorarum do not obtain significant amounts of organic carbon from seagrasses. There appears to be a clear trend for juvenile rather than adult penaeids to eat plant material (Hill et al. 1990).

Figure 18.43. Panulirus ornatus is one of the largest (> 3 kg wet weight) crustaceans on tropical reefs, and is an economically valuable species in Indonesian waters. Juveniles depend on seagrass beds for protection and/or food.
Photo courtesy of R. Steene, Cairns.
Molluscs. The molluscs are one of the best-known groups of seagrass-associated macroinvertebrates in Indonesia, and perhaps the most overexploited (fig. 18.45). In a number of temperate studies molluscs have been shown to be a very important component of the seagrass ecosystem, both in terms of biomass and role in the energy flow through the seagrass system (Watson et al. 1984). It was demonstrated that 20% to 60% of epiphyte biomass in Philippine seagrass meadows is utilized by epifaunal communities dominated by gastropods (Klumpp et al. 1992). However, their role in Indonesian seagrass ecosystems remains relatively unknown. The majority of molluscs in temperate seagrass beds are detrivorous, with very few feeding directly on the live seagrass (Kikuchi 1980). Gastropods tend to feed mainly on periphyton (Klumpp et al. 1989).
Mudjiono et al. (1992) recorded 15 species of molluscs (i.e., 11 gastropods and four bivalves) from the seagrass meadows in Ban ten Bay, southwest Java Sea. The rather impoverished mollusc fauna was collected from five different locations with seagrass associations ranging from monospecific Enhalus acoroides to a three-species association consisting of E. acoroides, Cymodocea serrulata and Syringodium isoetifolium, as well as a seven-species association comprising Enhalus acoroides, Cymodocea serrulata, C. rotundata, Thalassia hemprichii, Halodule uninervis, Syringodium isoetifolium and Halophila ovalis. In total, seven gastropod families (i.e., Trochidae, Cerithiidae, Strombidae, Muricidae, Columbellidae, Nassariidae and Fasciolariidae) and three bivalve families (i.e., Arcidae, Veneridae and Mactridae) were present in all associations (Mudjiono et al. 1992). Unfortunately, nothing of ecological significance can be learned from the abundance records, since the entire bay is heavily exploited, and three study locations were located within a short distance of a large village on Pulau Panjang. Only two gastropods were common to all locations, namely Pyrene versicolor and Cerithium tenellum. Note that the data presented by Mudjiono et al. (1992) are indicative of overexploitation of seagrass resources (e.g., only four juvenile, 3-5 mm diameter, Trochus niloticus were collected) and the effects of pollution (i.e., siltation) rather than ecological patterns.
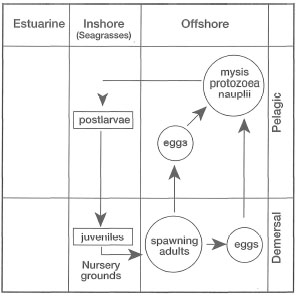
Figure 18.44. Life cycle of some penaeids that depend on seagrass beds for their postlarval and juvenile-stages.
Modified after Dall et al. 1990; 'Type 3' life cycle.
In a similar study conducted in Kotania Bay, west Seram, Wouthuyzen and Sapulete (1994) recorded 24 species from Osi Island, eight species from Pelitajaya village and 22 species from Kotania village. The type of seagrasses were not mentioned, however, they noted that several species of molluscs that were formally very abundant (i.e., Anadara antiquata, Pinna nobilis, P. muricata and Atrina vexillum) are now relatively rare, indicating overexploitation of these resources.
Better appreciation of the potential economic value of seagrass resources can be obtained from a recent study by Mudjiono and Sudjoko (1994), conducted in three bays on the south coast of Lombok. A total of 70 mollusc species were recorded from the seagrass beds, many of which are economically valuable. Among the more abundant gastropods were Pyrene versicolor, Strombus labiatus, S. luhuanus and Cymbiola vespertilio (fig. 18.46). Common bivalves were Anadara scapha, Trachycardium flavum, T. subrugosum, Peryglypta crispata, Mactra spp. and Pinna bicolor(Mudjiono and Sudjoko 1994). In addition, a number of Conus species were found as well as other economically valuable shells.
Echinoderms. The echinoderm (Echinodermata) fauna is the most conspicuous and economically valuable component of benthic seagrass communities (fig. 18.47). Five major classes of echinoderms are found in Indonesian seagrass ecosystems. In an order of decreasing economic importance they are: 1) Holothuroidea (sea cucumbers or teripang); 2) Echinoidea (sea urchins); 3) Asteroidea (sea stars); 4) Ophiuroidea (brittle stars); and 5) Crinoidea (feather stars). Of the five classes, the echinoids are the most important group in Caribbean seagrass ecosystems, since they are the main grazing group (Lawrence 1975, Greenway 1976). Other large echinoderms such as Protoreaster, Pentaceraster and Culcita spp. are predominantly detrivores or omnivores and do not graze directly on seagrasses. In Indonesia, as well as throughout much of the Indo-Pacific, echinoids, while present in seagrass beds (e.g., Tripneustes gratilla, Diadema setosum, etc.), are rather rare in many reefal environments. More common on intertidal reef flats are the horned sea stars Protoreaster nodosus and Linckia laevigata. P. nodosus is frequently very abundant in areas where sea cucumber populations have been heavily overexploited (e.g., Salabanka, Komodo). However, some echinoids can be locally very abundant, and frequently form large aggregations. Diadema setosum, in particular, aggregates during the day in shallow back-reef pools, where they often seek shelter against large coral colonies, if they are present. These aggregations are inactive during the day, but disperse in late afternoon to feed in the periphery. In the Caribbean, Diadema antillarum are known to graze heavily on Thalassia testudinum and Syringodium filiforme, and create "halos" between the base of the reefs and the outlying seagrass beds (Ogden et al. 1973). However, D. setosum has been classified as an omnivore (algivore/detrivore) (Guib 1981), thus their general role in seagrass trophodynamics remains unclear (Klumpp et al. 1993).
Figure 18.45. Seagrass beds are important mollusc habitats, often supporting species with economically valuable shells. A) Predatory gastropod Cassis cornutus partially burrowed in a sparse Halophila ovalis seagrass meadow on a gentle sand slope (10 m). This species feeds predominantly on echinoids (i.e., sea urchins); Sangalaki Island. B) Predatory Conus betulinus is a common inhabitant of seagrass meadows
Photos by Tomas and Anmarie Tomascik.
Figure 18.46. Cymbiola vespertilio (Volutidae) is a common predatory gastropod in seagrass beds where it feeds on other gastropods and bivalves. Captured prey is held within the foot of the animal and taken below to be consumed.
Courtesy of R. Steene, Cairns.

Figure 18.47. Echinoderms are a conspicuous benthic fauna in seagrass ecosystems. Diadema setosum (black sea urchin), Echinometra mathaei (urchin under rock, centre back) and Holothuria (Lessonothuria) verrucosa. Samama Island, East Kalimantan.
Photo by Tomas and Anmarie Tomascik.
Most echinoderms, with the exception of many holothuroids, feed at night. However, Klumpp et al. (1993) reported that Tripneustes gratilla and Salmacis sphaeroides feed (i.e., graze) continually throughout the day and night, without evidence of any periodicity. They were observed to forage close to the substrate, ingesting algae, seagrass debris and living seagrass fronds (Klumpp et al. 1993). Tripneustes gratilla shows a preference for attached fronds of Thalassia hemprichii and, to a lesser extent, Syringodium isoetifolium. A high percentage of actively grazing T. gratilla (77%-89%) feed on live seagrass fronds (Klumpp et al. 1993). The impact of T. gratilla grazing on seagrasses varies spatially as well as temporally, since sea urchin abundance and size vary seasonally. Sea urchin grazing rates are a function of both urchin densities and their body size (Klumpp et al. 1993). For example, Mukai and Nojima (1985) reported that Tripneustes gratilla in Papua New Guinea graze only 1.4% of seagrass production at densities of 0.1 individuals m-2. Klumpp et al. (1993) reported that between March and July, T. gratilla consumes 1%-5% of seagrass production, while between November and January the sea urchin consumption increases to more than 50% of seagrass production. They concluded that on an annual basis, T. gratilla consumes about 24% of total seagrass production, thus a large proportion of average seagrass frond production remains ungrazed. The fate of this large particulate organic carbon pool remains poorly understood, but it is either exported, or retained (i.e., deposited) within the system, where it is decomposed and remineralized. How much of this particulate organic matter is exported to adjacent systems or retained is still being debated. Nienhuis et al. (1989) believe that less than 10% of net production is exported from seagrass meadows, the rest being retained within the system.
In a recent study, Azis and Soegiarto (1994) recorded 45 echinoderm species in seagrass beds located in Kuta and Gerupuk Bays on the south coast of Lombok. All major groups were present: Asteroidea, Echinoidea, Holothuria, Ophiuroidea and Crinoidea (table 18.5). They noted that several economically important holothuroid species (Holothuria and Actinopyga) as well as the sea urchin Tripneustes gratilla have declined in abundance. Similar depletions in echinoderm populations were reported from Kotania Bay (west Seram Island, Moluccas), where sea-grass meadows formerly supported a high abundance of economically important holothuroids. In 1983, the extensive seagrass meadows in Kotania Bay supported high population densities (i.e., 1-2 individuals m-2) of nine economically important sea cucumber species, namely Bohadschia marmorata, B. argus, Holothuria (Metriatyla) scabra, H. (Microthele) nobilis, H. vagabunda, H. (Thymiosycia) impatiens, H. (Halodeima) edulis, Thelenota ananas, and Actinopyga miliaris. In a 1993 inventory of the same area, only three sea cucumbers were recorded within a distance of 500 m (Wouthuyzen and Sapulete 1994). Average body size of sea cucumbers decreased from c. 22 cm in 1983 to less than 15 cm in 1993. The decline of the stock and size are attributed to intensive collections by local people to supply the lucrative teripang (i.e., beche de mer) market. Another heavily overexploited echinoderm species whose populations have declined sharply during the past 10 years is the edible sea urchin Tripneustes gratilla.
Table 18.5. Common echinoderm (Echinodermata) fauna in the seagrass meadows along the south coast of Lombok, West Nusa Tenggara.
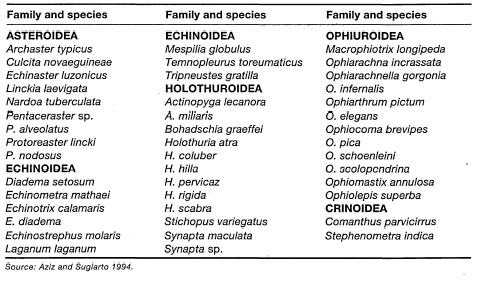
Seagrass Fish Fauna. Throughout their distributional range, seagrass ecosystems, whether small or large, are important habitats for a variety offish species (Kikuchi 1980; Pollard 1984; Bell and Pollard 1989). In their review of seagrass fish associations, Bell and Pollard (1989) identified seven major characteristics offish assemblages associated with seagrasses. With some modifications to Bell and Pollard (1989), the characteristics are as follows:
i) The diversity and abundance offish in seagrass meadows are usually higher than on adjacent bare substrata (e.g., sand, coral rubble, mud).
ii)The duration of the fish-seagrass association varies among species and life-cycle stages.
iii)The majority of fish associated with seagrass beds are recruited from the plankton, thus seagrass meadows are important nursery grounds for many commercially important species.
iv)Zooplankton and epifaunal crustaceans are a major nutrient pool for seagrass-associated fish, with plant, detrital and infaunal components of the seagrass food web being under-utilized by fish.
v) Vertical differences (i.e., resource partitioning) in species composition occur in most seagrass beds.
vi)Strong linkages exist between seagrass beds and adjacent habitats, the relative abundance and composition offish species in seagrass beds being dependent on the type (e.g., coral reefs, estuaries, mangroves) and proximity of adjacent habitats, as well as on day-night cycle.
vii)Fish assemblages from different seagrass meadows are often different, even when the two habitats are adjacent.
One of the first studies of seagrass-associated fish fauna in Indonesia was that of Hutomo and Martosewojo (1977). The study was conducted in Thalassia hemprichii and Enhalus acoroides seagrass meadows associated with a multi-lagoonal patch reef (Pari Islands) in the Kepulauan Seribu Complex. A total of 78 seagrass-associated fish species were collected during the study. However, out of the 32 fish families present, only six (Apogonidae, Atherinidae, Labridae, Gerridae, Siganidae and Monacanthidae) could be considered as an important resident group. Hutomo and Martosewojo (1977) classified the Pari Islands seagrass fish assemblages into four main categories as follows:
i) Full-time residents which spawn and spend most of their lives in seagrass beds (e.g., Apogon margaritophorus);
ii) Residents which spend their lives in the seagrass beds during their juvenile through adult life cycle, but spawn outside the seagrass beds (e.g., Halichoeres leparensis, Pranaesus duodecimalis, Paramia quinquelineata, Gerres macrosoma, Monacanthus tomentosus, Monacanthus hajam, Hemiglyphidodon plagiometopon, Sygnathoides biaculeatus);
iii) Residents which occur in seagrass beds only during their juvenile stage (e.g., Siganus canaliculars, S. virgatus, S. chrysospilos, Lethrinus spp., Scarus spp., Abudefduf spp., Monacanthus myHi, Mulloides samoensis, Pelates quadrilineatus, Upeneus tragula); and
iv) Occasional residents or transients that visit seagrass beds to seek shelter or food.
The 1977 study in the Pari Islands was followed by a long-term study of seagrass fish assemblages in Banten Bay, southwest Java Sea. The results from the Banten Bay study (Hutomo 1985) supported earlier views that only a small number of fish species permanently reside in seagrass beds. However, it was also reconfirmed that seagrass beds act as nursery grounds for many economically valuable fish species (fig. 18.48). Furthermore, species composition of seagrass fish assemblages was shown to be influenced by the species pool of adjacent communities. Hutomo (1985) reported that seagrass beds with higher densities of seagrass supported higher abundance of fish. The study also demonstrated that Enhalus acoroides meadows supported higher fish abundance than Thalassia hemprichii, whose blades are shorter. Rollon and Fortes (1991) obtained similar results, and found that high seagrass biomass was positively correlated with fish diversity (i.e., high number of fish and species composition). They also noted that Enhalus acoroides and Thalassia hemprichii were the dominant seagrass species. In contrast, mixed (high diversity) seagrass meadows dominated by shorter seagrasses support lower fish abundance and lower species richness. Rollon and Fortes (1991) suggested that seagrass beds act mainly as shelters where fish can avoid predation. Similar results were obtained by Sudara et al. (1992), basically reconfirming an earlier hypothesis that seagrass beds with greater structural complexity (i.e., larger plants with more surface area) and higher seagrass densities will support higher abundance of fish species and fish biomass (Heck and Ort 1980).

Figure 18.48. Seagrass-associated fish such as Lethrinus olivaceus (long-nosed emperor or katamba) spend their juvenile stage in seagrass beds and support subsidence fisheries when adults. Salabanka Islands, Central Sulawesi.
Photo by Tomas and Anmarie Tomascik.
The current species list of Indonesian seagrass-associated fish species stands at about 360, and is based on studies conducted between 1977-1991 (Hutomo and Martosewojo 1977; Hutomo 1985; Kiswara 1991). The studies demonstrated that significant differences exist in seagrass-associated fish fauna between different regions of the archipelago. Of the 360 species associated with seagrass beds, 78 were recorded in Kepulauan Seribu, 165 species were collected from Ban ten Bay and 205 species from west of Seram Island (Hutomo and Martosewojo 1977; Hutomo 1985; Peristiwady 1991). The differences are primarily a result of different ecosystems studied as well as a result of different sampling efforts. However, many economically important species, such as the siganids, were common to all large coastal seagrass beds (e.g., Banten Bay, Miskam Bay, Kotania Bay, KutaBay, etc.). This indicates that siganids rely on seagrass beds for both food and shelter. Lepiten (1994) has recently demonstrated that while grazing by the rabbitfish, Siganus canaliculars, channels relatively little primary production into the seagrass food chain, a significant portion of the seagrass eaten by the siganid population is passed on into the detrital food chain (fig. 18.49).
Peristiwady (1994a) used fish-gut analyses to determine the primary food sources for dominant seagrass-associated fish species. Based on the gut analyses information, Peristiwady (1994a) proposed a possible food web that links the high primary production of benthic primary producers to the top predators in the seagrass beds. Figure 18.50 illustrates that crustacean seagrass fauna is the dominant food currency of the south Lombok seagrass beds, which links the benthic primary production to higher trophic levels.
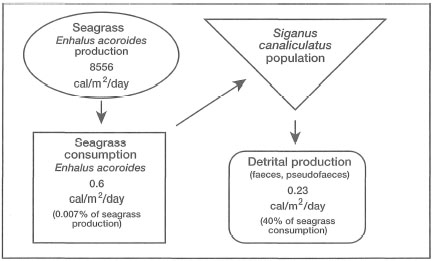
Figure 18.49. Daily energy flow based on grazing activity of Siganus canaliculatus population in Bais Bay, Philippines.
From Lepiten 1994. Enhalus production based on Estacion and Fortes 1988.
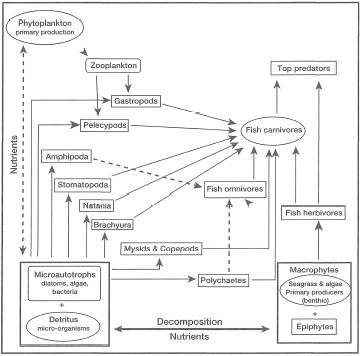
Figure 18.50. Diagram of major food pathways in a simplified seagrass food web. Solid lines linking the various faunal groups indicate main pathways, dashed lines indicate secondary pathways.
Modified from Peristiwadi 1994a.
Comparison ofFish Assemblages. There are few studies in Indonesia that can be used in a comparative ecological framework, however, some basic qualitative comparisons are possible. Observed differences in community structure of seagrass-associated fish faunas between different areas are related to the structure of seagrass communities and adjacent habitats as well as to substrate type and environmental conditions. A recent study in die Spermonde Archipelago, South Sulawesi, revealed that 49 fish species were associated with two different seagrass habitats (i.e., Gusung Talang, inshore seagrass beds on terrigenous substrate, and Barang Lompo, reef flat-associated seagrass beds) (Erftemeijer and Allen 1993). The total number offish species at Gusung Talang and Barang Lompo were similar, 27 and 26 species, respectively, however, the two locations did not share any common species. The majority of fish in Barang Lompo seagrass beds were species characteristic of reef flat environments. Nonetheless, three species at Barang Lompo were considered as typical of seagrass habitats, namely Acreichthys tomentosus, Novaculichthys macrolepidotus and Centrogenys vaigiensis. In Gusung Talang, the seagrass beds were dominated by more estuarine-type groups such as engraulids, clupeids and siganids, all commercially important species. The most recent work by Hutomo and Parino (1994) showed that 85 fish species were associated with mixed seagrass beds on the south coast of Lombok. The most common fish were Syngnathoides biaculetus, Novaculichthys sp., Acreichthys sp., and Centrogenys vaigiensis. A number of economically important species such as siganids, lethrinids, carangids and lutjanids were recorded in all mixed seagrass beds.
The reef-associated seagrass beds on the reefs of the Kepulauan Seribu patch reef complex support nine commercial fish species. In Banten Bay, which has a smaller area than Kepulauan Seribu, 12 commercial seagrass-associated fish species support an important local coastal fishery. Seagrass beds in Banten Bay are not reef-dependent, but in the past were mostly associated with mangroves which have been largely converted into fish ponds and agricultural lands. The main commercial fish species in Banten Bay seagrass meadows are listed in table 18.6. Most of the fish collected from the seagrass beds were juveniles and sub-adults. In Kotania Bay on the west coast of Seram, Peristiwady (1994a) recorded 8-9 commercial fish species from the reef-associated seagrass beds at Osi and Marsegu Islands. Seagrass-associated species represented 33.2% to 45.4% of total specimens collected from the two islands, respectively. Siganids were the most dominant species, representing about 5% of total number of individuals collected.
Ecological Characteristics
Processes and Functions. While seagrass ecosystems are not as well-known to the general public as coral reefs or mangroves, their ecological significance may be comparable, and in some parts of the archipelago even higher (e.g., Am Islands). Seagrass ecosystems have a number of important ecological attributes related to physical, chemical and biological processes. The role of seagrass beds in stabilization of coastal sediments by their extensive root/rhizome networks has been recognized for some time. In fact, artificial seagrasses are now being manufactured to protect economically valuable beachfront properties where pollution has destroyed natural seagrass beds. Seagrass beds have a modifying effect on both seaward and landward ecosystems. They shelter landward habitats (e.g., mangroves, beaches, sand dunes) by dissipating incoming wave energies, while seaward habitats, such as coral reefs, frequently benefit from the filtration effect provided by extensive sea-grass beds. Land runoff entering coastal waters through rivers is dissipated by coastal currents that can be modified by seagrasses. As water masses pass over sea-grass beds, current velocities are reduced, which, in turn, promotes rapid sedimentation of fine inorganic and organic particles.
Seagrass beds play a vital role in coastal nutrient dynamics. Seagrass sediments are characterized by high organic content with rapid nutrient recycling across the sediment/water interface. Seagrass beds play a key role in global sulphur and nitrogen cycles through sulphate reduction and nitrogen fixation. Surface and interstitial porewaters are oxygenated by extensive root/rhizome networks, creating an oxic zone (i.e., rhizosphere) which promotes aerobic decomposition and bacterial production. In fact, anaerobic and aerobic processes in seagrass sediments occur very close to each other, primarily as a result of oxygen diffusion from roots and rhizomes into the surrounding media (Moriarty and Boon 1989). It is generally accepted that nutrients and dissolved organic matter leak to adjacent systems, while other nutrients may be "filtered out" of ambient water through photosynthetic processes (benthic and pelagic).
Table 18.6. Size range of major economically important fish species found in Banten Bay seagrass beds.
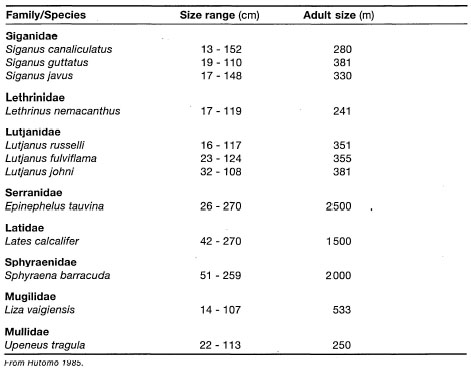
In terms of important biological attributes, seagrass beds maintain high gross primary production rates that are comparable to many other productive marine and terrestrial habitats (table 18.7). Much of the primary production in seagrass beds is attributed to the photosynthetic activity of seagrasses and their epiphytes. A major contribution of seagrasses to the nutrient dynamics of the system is through the detrital pathway, based on the decomposition of seagrass leaves. Live seagrass leaves are generally an underutilized resource. However, live leaves provide an extensive surface area for the attachment of epiphytes which considerably increase the productivity of the system. High productivity (i.e., food) and physical complexity (i.e., protection) are two key attributes that partly explain why seagrass beds are important nursery and feeding areas for a variety of organisms.
Research of ecological processes and functions of seagrass ecosystems is a new and rapidly expanding field in Indonesia. Seagrass ecosystems are a major source of photosynthetically fixed organic carbon that enters coastal food webs by a variety of pathways through microbial decomposition of detrital matter, animal grazing and predation. Our understanding of major energy pathways through the system, as well as the functional linkages between seagrass beds and economically valuable coastal penaeid and finfish fisheries, is essential for the future management and conservation of these renewable resources. Figure 18.51 illustrates the complexity of interactions in a typical Philippine seagrass bed, which may be applicable to Southeast Asian seagrass ecosystems in general (Fortes 1990).
Seagrass ecosystems are not isolated entities, but interact with adjacent ecosystems in a complex manner through several mechanisms. The importance of ecological interactions between tropical coastal ecosystems (i.e., coral reefs, seagrass beds and mangroves) has attracted considerable attention (Ogden and Gladfelter 1983). Based on research conducted in the Caribbean, Ogden and Gladfelter (1983) identified five types of ecosystem interactions and connections between seagrass meadows, coral reefs and mangrove forests (fig. 18.52). The general concept is applicable to the Indo-Pacific, however, specific linkages and pathways between these ecosystems have not been quantified.
Table 18.7. Comparison of estimates of primary production of selected terrestrial and marine ecosystems. Net P: net primary production. Values given in g C.m-2.year-1.
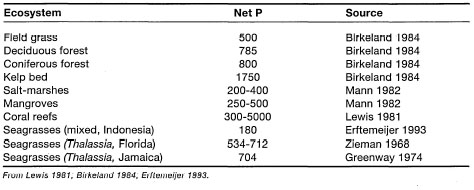
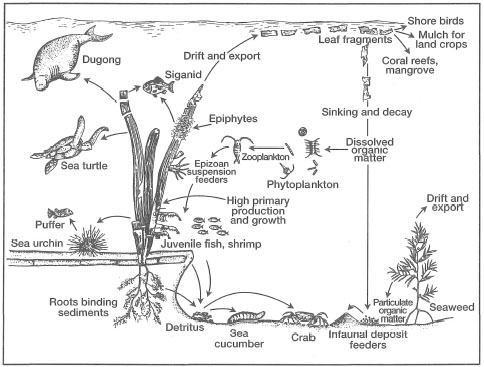
Figure 18.51. Simplified food web of a Philippine seagrass ecosystem.
From Fortes 1990.
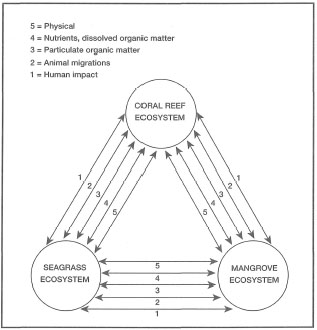
Figure 18.52. The main types of interactions that occur between coral reef ecosystems, mangrove ecosystems and seagrass ecosystems.
From UNESCO 1983.
Seagrass Productivity.
NUTRIENTS AND LIGHT. Seagrass beds are dynamic systems occupying a variety of habitats, each under a unique set of environmental conditions. It is, therefore, not surprising that there is considerable variation in primary productivity rates and biomass between seagrasses in different parts of the world, and among different areas within a particular region (Hillman et al. 1989). Primary productivity of sea-grasses is dependent on two major environmental factors, namely the availability of solar energy and nutrients. In general, light controls the vertical (i.e., depth) distribution of seagrasses, and thus environmental conditions that affect light attenuation through seawater will be of importance. Seagrasses occurring in turbid coastal waters have much shallower depth distributions than seagrasses in clear-water environments. However, nutrients are the key factor that in fact determines the productivity of seagrasses. It is expected that highly productive systems, such as seagrasses, will be nutrient-limited either by nitrogen or phosphorus. Phosphorus is most likely the limiting nutrient, since nitrogen fixation within seagrass beds can produce sufficient quantities of usable nitrogen to maintain high production rates. Note that seagrasses, unlike most macrophytes, can tap two dissolved nutrient pools, the interstitial water surrounding their roots and rhizomes, and the seawater in the water column. Seagrasses are able to absorb phosphorus directly from seawater through their leaves, or from sediments through their roots (McRoy and Barsdate 1970; Brix and Lyngby 1985). Erftemeijer (1993) found that nutrient concentrations in the porewater were significantly higher than in the water column.
There are few long-term studies on nutrient limitation and its role in structuring seagrass communities in the archipelago (Erftemeijer 1993). In turbid coastal waters influenced by terrestrial processes, nutrient limitation will seldom limit seagrass production. Erftemeijer (1993) demonstrated that in a number of inshore seagrass beds affected by river runoff, nutrient limitation was not a major factor influencing seagrass productivity. Excessive nutrient subsidy resulting from land runoff is often more deleterious to coastal seagrass beds than nutrient limitation, since high influx of nutrients will often result in eutrophication which, among other things, reduces the amount of light reaching the seagrasses through reduction in water clarity and increased growth of epiphytes. Even in oceanic settings (i.e., atoll lagoons), nutrient limitation may not be a significant impediment to seagrass productivity, since seasonal upwelling, as well as daily tidally-induced upwelling, pump significant quantities of nutrients into these systems (T. Tomascik, pers. obs.). Nitrogen limitation will most likely not be a factor, since nitrogen is delivered to seagrass beds through nitrogen fixation which occurs within the rhizosphere of seagrass root/rhizome networks, as well as by advective input from external sources (Patriquin and Knowles 1972). Note that concentrations of dissolved nitrogen in seagrass sediments are quite low, considering the high influx of organic matter, which indicates rapid recycling (i.e., uptake, turnover) (Barnes and Mann 1980). Seagrass beds associated with reef flat environments will also benefit from nitrogen fixation that occurs in algal turf zones on the windward reef flats. Note that nitrogen fixation on reefs probably occurs throughout the reef ecosystem (Hatcher 1985; Wiebe 1985).
A number of studies have indicated that phosphorus is the main limiting nutrient in most marine environments (Smith 1984; Smith and Atkinson 1984; Atkinson 1992,1993). Phosphate is delivered to the seagrass system through advective processes. Dissolved inorganic phosphate removed from the water column by the growth of organisms (e.g., phytoplankton) is buried in seagrass sediments where it accumulates as organic and inorganic phosphorus (Atkinson 1987). Under aerobic conditions, most of the ammonium and phosphate ions present in seagrass sediments are adsorbed to ferric iron (Barnes and Mann 1980). In coastal waters with high inputs of organic matter, phosphate concentrations in sediment pore-water largely determine the relative concentrations of phosphate in different sediment components (Atkinson 1987).
As organic matter in sediments undergoes mineralization (i.e., decomposition), phosphate is released into interstitial porewaters where it becomes available for absorption through the seagrass root system. Sulphate reduction in anoxic sediments is one mechanism through which phosphorus becomes available to the seagrasses. During sulphate reduction, iron phosphate is reduced from the ferric to ferrous form, thus releasing phosphate and dissolved ammonia in the process. It has been suggested that interstitial porewater contains sufficient phosphorus concentrations to maintain seagrass production for up to three years (Patriquin 1972). The upper layers of seagrass sediments have often high concentrations of reactive phosphorus (Moriarty and Boon 1989). Seagrasses are able to draw on these phosphate reserves through their root/rhizome mats, and subsequently leak part of this phosphate pool to adjacent systems, mainly through export of their leaves. In addition, the extensive root/rhizome mats that occupy the upper sediment layers release considerable amounts of oxygen into the rhizosphere, thus keeping the upper sediment surface oxygenated. Aerobic conditions seem to facilitate slow but continuous release of phosphorus into the overlying water, which transports some fraction of this nutrient pool to adjacent waters (Barnes and Mann 1980).
It has been suggested that phosphorus is particularly limiting in seagrass beds on calcareous sediments (Birch et al. 1981; Smith 1984; Short 1987). This may occur because phosphate is known to coprecipitate with CaCO3 or CaPO4 of shells, tests, and bones of marine organisms. Thus, CaCO3 production will cause phosphate deposition (Atkinson 1987). It may therefore not be surprising to find phosphate limitation in reef-associated seagrass beds. However, Erftemeijer (1993) found that porewater phosphate concentrations in a reefal environment were surprisingly high (> 10 μm). In fact, they were higher than in a coastal habitat where high productivity was fueled by terrestrial runoff. Erftemeijer (1993) attributed the anomalous phosphate concentrations to rapid regeneration of both N and P in the rhizosphere and subsequent removal of ammonia by nitrification. High phosphate concentrations were also maintained by the poor adsorptive capacity of the coarse-grained sediments.
Light can be an important factor limiting seagrass production and distribution in shallow-water coastal environments (Erftemeijer 1989; Verheij and Erftemeijer 1993). Vertical (i.e., depth) and horizontal distribution of seagrass beds in shallow-water coastal habitats dominated by terrigenous sediments are mainly light-dependent. Seagrass beds located in close proximity to the mainland are often influenced by salinity fluctuations, nutrient pulses, increased turbidity, and subsequent light limitation. Figure 18.53 provides seasonal water-column nutrient profiles in seagrass beds in two contrasting environments in the Spermonde Archipelago. Barang Lompo is an offshore, reef-associated, seagrass community, while Gusung Tallong is located in an inshore environment frequently influenced by freshwater runoff, off mainland Sulawesi. Lower shoot densities and biomass, high epiphyte cover, increased leaf length and elevated nitrogen and phosphorus content in seagrass plant material are all related to considerable nutrient subsidy (Erftemeijer 1993). It was demonstrated that the morphology of certain seagrass (and macroalgae) species is nutrient-dependent. For example, leaf size of Enhalus acoroides is significantly larger in inshore seagrass habitats subjected to nutrient subsidy (i.e., river runoff) than in offshore reef-associated seagrass beds (Verheij and Erftemeijer 1993). In general, seagrass beds associated with offshore carbonate sediments (i.e., reef-derived) are subjected to less severe environmental fluctuations, and consequently, seagrass biomass shoot densities are higher, and epiphyte cover is significantly reduced (Erftemeijer 1993).
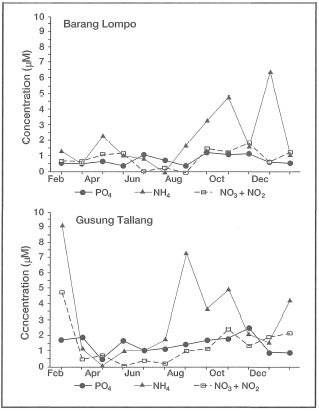
Figure 18.53. Seasonal fluctuations in monthly mean nutrient concentrations (soluble inorganic phosphate, ammonia and nitrate+nitrite nitrogen) at two contrasting seagrass habitats. Barang Lompo is an offshore seagrass bed associated with a patch reef, while Gusung Tallang is an inshore seagrass bed frequently influenced by river runoff.
From Erftemeijer 1993.
Seagrass Biomass and Density Estimates. A variety of techniques are currently used to measure seagrass biomass distributions. If measured over time (e.g., one year), biomass measurements are direct estimates of net production of the seagrasses, since they measure biomass accumulation. In the simplest case, biomass (B) is the net growth of organisms that remains after respiration (R) is accounted for. Thus, measuring change in biomass over a specific time interval (t) gives an estimate of net productivity (N) (Kemp et al. 1990). This relationship can be expressed by the following simple equation:
Gross productivity (G) = N + R
or
N = G-R
The above relationships, however, underestimate the net production of the system, since losses due to excretion, death and decomposition or grazing by herbivores are not included (Kemp et al. 1990). According to Kemp et al. (1990), the best estimate of seagrass net production is:
N = ΔB/Δt + E + D + H
where ΔB/Δt = change of biomass (B) over known time interval (t) plus losses due to excretion (E), death and decomposition (D) and removal by grazing animals (H).
'Biomass' refers to the above- and below-ground plant material, and is usually expressed as grams dry weight per square metre (g DW.m-2); sometimes ash-free dry weights (ADW or AFDW) are given (Nienhuis et al. 1989). 'Standing crop' refers to above-ground plant material only, and is the most frequently given production parameter estimate. Note, however, that many species invest more energy into growth of their below-ground biomass. For example, the below-ground rhizome biomass of Enhalus acoroides is 6-10 times larger than its above-ground biomass (Nienhuis et al. 1989). Cymodocea serrulata, C. rotundata and Halodule uninervis are pioneer species, and tend to invest more in below-ground biomass growth in established (stable) mixed vegetation beds than in monospecific (pioneer) beds (Nienhuis et al. 1989). In general, species characteristic of "climax" meadows (i.e., Thalassia hemprichii, Enhalus acoroides and Thalassodendron ciliatum) invest two to four times more energy into below-ground biomass growth than the colonizing species (e.g., Halodule uninervis, Cymodocea rotundata and C. serrulata) (Nienhuis et al. 1989). Biomass values show high variability due to differences in habitat (e.g., substrate type, turbidity, etc.), seagrass composition, seagrass densities and sampling techniques (Zieman and Wetzel 1980). Table 18.8 provides an example of some seagrass biomass values from different parts of the archipelago. As is clearly apparent, there is as much biomass variability between different regions (i.e., Banten Bay and Kuta Bay) as within a particular locality (i.e., Kuta Bay vs. Gerupuk Bay). In general, average biomass densities in seagrass beds vary between 1 g DW.m-2 to 2479 g DW.m-2.
Another frequently used measure of biomass estimate is seagrass density, usually expressed as number of shoots per m-2 (table 18.9). Results from various studies have demonstrated that average seagrass densities in seagrass beds range from 10 shoots.m-2 to 5600 shoots.m-2, a considerable variation, indeed. Brouns and Heijs (1991) estimated that, with some exceptions (e.g., Thalassia hemprichii and Halodule uninervis), variability of the density in monospecific beds is roughly a factor of two for most seagrasses. Kiswara (1994a) found that the density in H. uninervis in seagrass beds depended on the phenotype present (i.e., normal shoots vs. thin shoots). In Gerupuk Bay, south Lombok, H. unineruis densities ranged from 870 "normal" shoots.m-2 to 6560 "thin" shoots.m-2 within the same seagrass bed. Nienhuis et al. (1989) reported that Halodule unineruishad the highest density variability of all sea-grass species in mixed as well as in monospecific seagrass beds (table 18.10). In sea-grass beds where foliage covers more than 70% of the substrate, density of sea-grasses frequently depends on the species composition of the community and the relative age of the seagrasses (among other factors). In some species, such as Thalassia hemprichii, biomass is frequently a function of shoot density and total leaf area per leaf cluster (Brouns and Heijs 1991).
Seasonal studies of seagrass biomass and shoot densities in Indonesian waters are scarce (Erftemeijer 1993). However, it has been demonstrated that significant seasonal fluctuations in seagrass biomass do occur, and that strong correlations between seasonal fluctuations of environmental and biological variables exist (Erftemeijer 1993). In offshore seagrass beds, seagrass biomass appears to be a strong function of tidal exposure and water motion (i.e., waves and currents). Surprisingly, however, no significant correlation was found between seasonal variations in environmental variables associated with riverine discharge and seagrass biomass production (Erftemeijer 1993).
Photosynthetic Production Estimates. Carbon fixation through photosynthesis and organic matter accumulation through plant growth constitute the very basis for seagrass ecosystems, their physical structure, food supply and mineral cycles (Kemp et al. 1990). Primary production is an ecological equivalent to photosynthesis, which is CO2 fixation by plants through absorption of light energy. Two types of primary production are distinguished: 1) gross primary production; and 2) net primary production. These two are related by the simple equation:
NP = GP-Ra
where NP = net production; GP = gross production, which is total photosynthesis; and Ra = respiration of the autotrophs (Woodwell 1980). Thus, net primary production is the amount of energy left after accounting for the respiration (i.e., energy consumption during metabolism) of autotrophs. Units measured can be energy, dry organic matter or carbon (Woodwell 1980).
Table 18.8. Average biomass of seagrasses (g DW.m-2) at various locations throughout the Indonesian Archipelago.
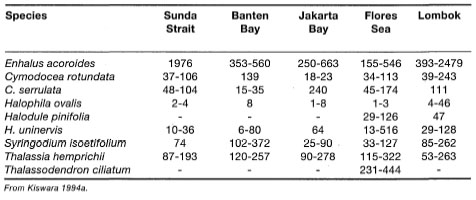
The oxygen (O2) evolution (i.e., photosynthesis) technique, using "bell jar" enclosures to estimate seagrass primary production rates, was employed in two Indonesian studies (Lindeboom and Sandee 1989; Erftemeijer 1993). The technique relies on plexiglass enclosures (bell jars) placed over seagrasses, and changes in O2 concentrations over time measured under light (i.e., gross photosynthesis) and dark (respiration) conditions. To convert oxygen evolution and consumption to carbon fixed and mineralized, a conversion factor of 0.29 was applied in both studies. Lindeboom and Sandee (1989) demonstrated that gross primary production rates of various seagrass communities in the Flores Sea vary from 1230 mg Cm-2.day-1 to 4700 mg Cm-2.day-1, while seagrass respiration consumption rates were between 860 mg C.m-2.day1 to mg Cm-2.day-1. Lindeboom and Sandee (1989) concluded that net primary production rates of seagrass communities in the Flores Sea vary between 60 mg Cm-2.day-1 to 1060 mg C.m-2.day-1, which translates to a maximum annual net primary production of about 387 g Cm-2, assuming the same rates of production throughout the year as during the study period (October). In contrast, the net primary production attributed to epiphytes alone accounts for a maximum annual net primary production of about 84 g Cm-2 of leaf surface area (Lindeboom and Sandee 1989). Thus, epiphytes contribute up to 36% to the net primary production rates in seagrass communities. Surprisingly, maximum annual net primary production rates of organic carbon on barren sediments were up to 65.7 g Cm-2, which is very close to the net primary production rates of most seagrass communities.
In a comparative study of two different seagrass environments in the Spermonde Archipelago, Erftemeijer (1993) obtained very similar results using the bell-jar technique. Gross primary production rates ranged from 900 mg Cm-2day-1 to 4400 mg Cm-2.day-1. Interestingly, the bell-jar technique used in the Spermonde Archipelago did not reveal any significant differences in seagrass primary production rates between coastal and reefal environments. Net primary production was slightly negative in a number of stations (i.e., 7 out of 12) and was generally below 500 mg Cm".day. Low net primary production rates were attributed to high community oxygen consumption rates. Respiration and mineralization rates of seagrass communities (which include seagrasses, epiphytes, epifauna, benthic algae, macro-, meio- and microfauna and bacteria) indicate that most of the gross carbon production is consumed within the system. Higher net primary production rates were obtained from monospecific stands of Thalassia hemprichii, where combined seagrass and epiphyte net production rates reached 1.5 to 1.9 g C.m-2.dayl (maximum 694 g C.m-2.yr-1). Nienhuis et al. (1989) suggested that seagrass communities are self-sustaining systems and export very little of their photosynthetically fixed carbon to adjacent ecosystems such as coral reefs. The results obtained from the Spermoncle Archipelago seem to support this general hypothesis. However, Erftemeijer (1993) points out that many seagrass communities (58% of his study sites) seem to use more energy than is actually produced by the autotrophic seagrass community (i.e., negative net production value). This suggests that, while recycling of nutrients and organic carbon is high, seagrass beds may not be as self-sustaining as earlier suggested by Nienhuis et al. (1989). Filter- and suspension-feeding macroinvertebrates constitute a significant consumer component of the seagrass community. Seagrass beds are not isolated systems, but are linked with adjacent oceanic and coastal ecosystems through ocean currents. Seagrass beds are continually being "flushed" by fast-flowing currents that bring with them considerable amounts of allochthonous organic material (plankton, zooplankton) which are available to the heterotrophic seagrass community. This external connection has not been investigated in Indonesian waters, yet it may be of considerable importance, especially with regards to coral-reef-associated seagrass beds.
Table 18.9. Average density of seagrasses (shoots.m-2) at various locations throughout the Indonesian Archipelago.
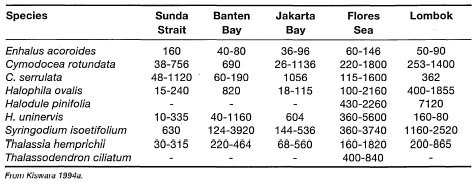
Leaf-Marking Method Estimates. Leaf production over time is frequently measured by the leaf-marking technique and in situ monitoring of leaf elongation (Dennison 1990). Leaf-marking relies on the concept of 'plastochrone', which is defined slightly negative in a number of stations (i.e., 7 out of 12) and was generally below 500 mg Cm-2.day-1. Low net primary production rates were attributed to high community oxygen consumption rates. Respiration and mineralization rates of sea-grass communities (which include seagrasses, epiphytes, epifauna, benthic algae, macro-, meio- and microfauna and bacteria) indicate that most of the gross carbon production is consumed within the system. Higher net primary production rates were obtained from monospecific stands of Thalassia hemprichii, where combined seagrass and epiphyte net production rates reached 1.5 to 1.9 g C.m-2.day-1 (maximum 694 g C.m-2.yr-1). Nienhuis et al. (1989) suggested that seagrass communities are self-sustaining systems and export very little of their photosynthetically fixed carbon to adjacent ecosystems such as coral reefs. The results obtained from the Spermoncle Archipelago seem to support this general hypothesis. However, Erftemeijer (1993) points out that many seagrass communities (58% of his study sites) seem to use more energy than is actually produced by the autotrophic seagrass community (i.e., negative net production value). This suggests that, while recycling of nutrients and organic carbon is high, seagrass beds may not be as self-sustaining as earlier suggested by Nienhuis et al. (1989). Filter- and suspension-feeding macroinvertebrates constitute a significant consumer component of the seagrass community. Seagrass beds are not isolated systems, but are linked with adjacent oceanic and coastal ecosystems through ocean currents. Seagrass beds are continually being "flushed" by fast-flowing currents that bring with them considerable amounts of allochthonous organic material (plankton, zooplankton) which are available to the heterotrophic seagrass community. This external connection has not been investigated in Indonesian waters, yet it may be of considerable importance, especially with regards to coral-reef-associated seagrass beds. as the time interval between development stages of the plant (e.g., time period between the initiation of two leaves) (Dennison 1990). Leaf-marking methods (hole punching and plastochrone) have been used in seagrass meadows at Taka Bone Rate Atoll (Brouns 1985), Kepulauan Seribu (Azkab 1988a,b), Banten Bay (Moro 1988) and, most recently, in Lombok (Azkab and Kiswara 1993) and the Spermonde Archipelago (Erftemeijer 1993). Production rates obtained from these studies are expressed as growth rate per unit time (e.g., mm.day-1), production (e.g., g DW.m-2.day-1) or plastochrone interval per day.
Table 18.10. Average shoot density of seagrass species in mixed and monospecific seagrass meadows in the Flores Sea. In all sampling locations foliage cover is >70%, except for Thalassodendron ciliatum (>50%). (SD in parentheses).
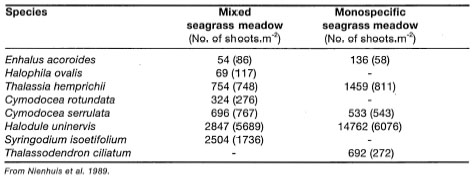
Erftemeijer (1993) was able to show that while primary production rates, based on oxygen evolution, were not sensitive in distinguishing differences between sea-grass beds in coastal and reefal habitats, the leaf-marking technique revealed significantly higher leaf growth rates in Enhalus acoroides in coastal muddy habitats than in the offshore reefal habitats (i.e., 3.1 mm.day-1 and 1.6 mm.day-1, respectively). Similar results were obtained for Thalassia hemprichiiTable 18.11 summarized the known growth rates of seagrass species from various locations in the archipelago.
Export of Organic Material. Rapid decomposition of plant material through bacterial and microbial action produces high quantities of detrital material, which fuels the main pathway through which primary production is channeled to higher trophic levels. The organic material produced from the decaying seagrass leaves, and other plant tissue, supports a complex detrital-based food web dominated by benthic deposit-feeders. While the detrital material is consumed by a variety of deposit-feeding benthos, the particulate organic matter in the water column is a major food source for filter-feeding organisms. How much of the seagrass production is retained within the seagrass system and how much is exported to adjacent systems is still a matter of debate. Floating seagrass leaves and shoots in offshore waters are a common occurrence in many parts of the archipelago with significant seagrass beds. However, it has been suggested that the loss of organic material (i.e., export) from Indonesian seagrass beds to adjacent systems is most likely less than 10% of net production (Nienhuis et. al. 1989). Note, however, that this estimate was based on purely qualitative observations. Birkeland (1985) proposed that up to 90% of the organic carbon fixed during primary production in tropical seagrass meadows enters the local food web as particulate organic material (POM).
Table 18.11. Average growth rates (mm.day-1) of seagrass leaves using leaf-marking techniques. Production rates in parentheses (g DW.m-2.day-1).
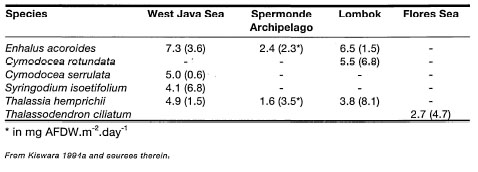
No efforts have been made, thus far, in Indonesia to quantify the export of organic material (or energy) from seagrass meadows to adjacent systems. As a result, qualitative value given by Nienhuis et al. (1989) has been taken at face value, and is often used to compare Indonesian systems with others. The islands of the Tukang Besi Archipelago, and the extensive lagoonal patch reefs in Taka Bone Rate Atoll, are all linked together by strong currents, and thus there is continual import and export of organic material from these systems. These have, however, not been quantified. Atolls such as Taka Bone Rate and Kaledupa, as well as reefs in the Banggai Islands, have extensive monospecific seagrass meadows on their reef flats and in the lagoons. During ebb-tide, surface currents on these reefs can be extremely high, which results in almost total removal of seagrass material from the seagrass beds. Obviously, in sheltered environments most of the seagrass biomass is turned into detrital matter and mixed with sediments where it supports a rich benthic fauna. This, however, is not the case in areas influenced by high-velocity currents. At Sago Island, Banggai Islands, a 200-300-m-wide and about 3-5-km-long "seagrass raft" (i.e., broken, decaying leaves and other plant material) was observed to "stream" from the barrier reef. The reef crest of the barrier reef was covered by a dense meadow of Thalassodendron ciliatum which provided all the material for the raft. While no detrital matter was visible in the Thalassodendron meadow, the reef slope was covered by a mat of decaying T. ciliatum leaves to a depth of 45 m. How much of the net production was being exported in the long Sago raft, or what was the biomass of leaves on the reef slope, are questions in need of some answers.
Natural Environmental Stresses
Fortes (1988) stated that cyclones, volcanic eruptions, tsunamis, pests, diseases, as well as seagrass population and community interactions (e.g., grazing and competition) are natural stresses in all tropical coastal ecosystems, including seagrasses. In the Southeast Asian region, destruction of seagrasses by cyclones, tidal waves and other natural phenomena remain largely unquantified. Tambora and Krakatau eruptions may have had considerable impact on a number of coastal ecosystems within the eruption impact zone, where large areas of shelf remain smothered by volcanic ash. Nienhuis et al. (1989) noted that areas covered by dark terrigenous sands (Tambora erupted in 1815) were generally devoid of seagrasses, suggesting that volcanic ash and sand may not be suitable seagrass substrates.
Cyclone damage to seagrasses is well-known in Australia, where whole meadows can be destroyed and large-scale seagrass distributional changes can occur (Hatcher et al. 1989; Taylor 1992). Storms can transport large amounts of seagrass biomass to the shore or adjacent ecosystems. Taylor (1992) reported that two years after major storm damage occurred, damaged seagrass beds adjacent to undisturbed seagrass beds began to recover due to the availability of seed stock. In contrast, recovery did not start in damaged areas that were far from undisturbed seagrass beds.
Herbivory accounts for only 10% to 15% of live seagrass consumption, with most of seagrass primary production being channeled into the detrital food web (Hatcher et al. 1989). Among some of the more important herbivores consuming live seagrass in Indonesia are the green sea turtles (Chelonia mydas), dugongs (Dugong dugori) and a variety offish species (e.g., siganids). In eastern Indonesia, dugongs have been observed to feed at high tide in intertidal meadows of Halodule, Halophila and Cymodocea, consuming both leaves and the root/rhizome mats (De Iongh, pers. comm.). In Papua New Guinea, Hattori et al. (1985) found that sea urchins consumed about 1.4% of daily seagrass production.
Very little is known about seagrass diseases, but they can be locally very destructive (Sloan 1993a). Generally, seagrass communities are regarded as relatively stable systems on time scales measured in 10 years and spatial scales of 10 to 1000 km2 (Larkum and den Hartog 1989). Few natural disturbances can perturb large seagrass systems to a point where their demise may be imminent, and disease is one of the most important. Along temperate North Atlantic coasts, a devastating "wasting disease" in the 1930s removed large areas of subtidal Zostera marina meadows (Rasmussen 1977; Giesen et al. 1990). The disease, caused by a slime mold (Myxomycete), was believed to have been caused by environmental stresses related to eutrophication, siltation, etc. However, den Hartog (1987) disputed earlier explanations as being too simple, and believed that a number of environmental and biological factors acting in synergy in a cyclical fashion may have been responsible. Large-scale community shifts in Zostera meadows reported in a number of studies have been associated with the presence of fungal pathogens (e.g., Labyrinthula) (Larkum 1977; Kirkman 1978; Orth 1979). Thus far, large-scale ecosystem-wide shifts have not been observed in Indonesian or Southeast Asian waters.
Anthropogenic Stresses
Zieman (1975) stated that: "Seagrass beds are especially vulnerable to degradation by human-induced stresses as they inhabit only the shallow marginal seas where the activities of man are the greatest". The extensive seagrass meadows in Indonesia have only recently attracted the interest of scientists. Currently, there are only a few documented cases showing that large-scale seagrass destruction has occurred due to anthropogenic impacts. However, anecdotal information from Kepulauan Seribuand Ban ten Bay, West Java, suggests that damage to Syringodium isoetifolium and Enhalus acoroides meadows has occurred as a result of increased turbidity caused by deep-draft motorized fishing vessels that now fish in the shallow coastal waters. In addition, Kiswara (1994b) has recently estimated that industrial development and land reclamation in Ban ten Bay resulted in a loss of about 35% of all seagrass meadows in the bay. In addition to the large-scale anthropogenic impacts (acute or lethal stresses), easily detected and observed, seagrass ecosystems are particularly vulnerable to low-level impacts or chronic stresses. 'Stress' is defined here as 'a measurable alteration of a physiological (or ecological) steady-state which is induced by an environmental change (natural or anthropogenic), and which renders the individual (or the community, or the ecosystem) more vulnerable to further environmental stress' (Bayne 1975). Chronic stress weakens the ability of seagrass communities to withstand and/or regenerate from natural disturbances such as diseases or storms. The following is a brief summary of anthropogenic activities with high potential to cause significant damage to Indonesian seagrass meadows. The assessment is based on existing studies from different regions of the world as reviewed by Sloan (1993a).
Coastal Works. Physical disturbances associated with nearshore dredging and filling activities are considered by many to be the greatest threat to tropical seagrass ecosystems (Zieman 1975; Hatcher et al. 1989; Aleem 1990). Similar conclusions have been reached in Indonesia, where siltation from coastal activities, upland deforestation, agriculture, and construction is considered one of the greatest single marine pollution threats (Soegiarto 1981). Seagrasses are able to tolerate some degree of siltation, turbidity, and burial, since they have evolved in depositionary environments in which fine sediments (organic and inorganic) are captured and bound, and from which sediments are also generated (i.e., production of calcareous epiphytes) (Sloan 1993a). Excessive siltation, however, robs plants of light through increasing water turbidity and direct settlement on seagrass leaves, leading to their degradation. Significant losses of coastal ecosystems in the densely populated western regions of the archipelago may be attributed to rapidly growing coastal populations as well as unregulated coastal development fueled by economic growth.
Industrial and harbour development activities in Ban ten Bay are a major threat to the extensive seagrass meadows found throughout the bay area. Significant degradation of seagrass beds has occurred since 1990 as a result of a large-scale reclamation project for industrial estates and harbour development. The decline of seagrass beds was first observed at Grenyang Bay and Bojonegara, where 80% of seagrass meadows were lost. These losses represent about 30% of the total seagrass area in Banten Bay. Most of the damage is attributed to large-scale sand mining and reclamation works that have significantly increased sedimentation rates and water turbidity. Increased fishing pressure from motorized boats has added a new dilemma to the management of this area. The use of motorized boats has increased fish landings, however, the damage that is being done to the seagrass beds by propellers (i.e., increase in turbidity) and beach seines (i.e., uprooting seagrasses) will inevitably lead to the loss of seagrass beds and a collapse of the fishery (Kiswara 1991).
Eutrophication. Tomascik and Sander (1985, 1987a,b) have demonstrated that anthropogenic eutrophication is a serious threat to tropical coastal ecosystems. Hundreds of square kilometres of seagrass meadows along the coastlines of large islands are under the direct influence of terrestrial processes (i.e., land runoff). Indonesian rivers are the major point sources of pollution, discharging immense quantities of terrigenous sediments frequently mixed with pollutants (e.g., herbicides, pesticides, etc.) into coastal waters (fig. 18.54). Domestic effluents in densely populated areas are a major source of nutrients. While water-quality standards are being developed for large- and medium-scale industries in an effort to reduce discharge of industrial pollutants, very little is being done about domestic waste disposal (e.g., sewage treatment plants).
Since many seagrass meadows are located in close proximity to human population centres (e.g., Banten Bay, Kepulauan Seribu) and along intensively cultivated padi-covered coastal plains, untreated sewage, contaminated ground water and agricultural runoff are the three main sources of nutrient subsidy (i.e., phosphorus and nitrogen) for coastal waters. These two macronutrients are essential for normal system function, and under natural conditions they often limit rates of primary production. Increased input of nutrients into seagrass meadows may initially stimulate production and growth, but, only up to certain concentrations. Compared to coral reefs, seagrass ecosystems operate at higher nutrient levels (Ogden and Gladfelter 1983). This is too often misinterpreted as higher tolerance to eutrophication. High nutrient concentrations in the water column stimulate phytoplankton production, frequently causing "blooms", and epiphyte growth on leaves. Increase in plankton and epiphyte biomass ultimately leads to a reduction in the light intensity that reaches the seagrasses and causes a reduction in seagrass photosynthesis and growth. It has been documented that eutrophication has already caused significant damage to a number of Australian seagrass meadows (Sheperd et al. 1989; Pearce 1991). Increased nutrient availability in the water column usually leads to a decline in seagrass biomass and a gradual takeover by macrophytes (Burkholder et al. 1992).

Figure 18.54. Deforestation and land clearing for agriculture in many regions of the archipelago has increased sediment loads of once clear-running rivers. Shallow-water coastal habitats such as seagrass beds and coral reefs are highly susceptible to siltation and sedimentation stress associated with terrestrial runoff.
Photo by Tomas and Anmarie Tomascik.
Industrial Effluents. With the exception of heavy metals, relatively little is known about the impacts of industrial effluents on tropical seagrass meadows in Indonesia. Bioaccumulation of heavy metals from sediments and ambient seawater by sea-grasses has been a well-known process for some time (Zieman 1975). Bioaccumulation of heavy metals occurs mainly in seagrass leaves. Seagrass mortalities due to heavy-metal pollution have not been widely reported, even though seagrass beds nearest to urban centres (e.g., Jakarta Bay) show high levels of heavy-metal contamination. Since seagrasses are a nutrient pool for a wide range of marine organisms, heavy metals are transferred to, and bioaccumulate, in organisms in higher trophic levels (e.g., prawns, fish) (Kiswara et al. 1990). In the relatively unpolluted waters of eastern Indonesia, heavy-metal concentrations (Cu/Cd/Pb/Zn) in seagrass leaves were low (Nienhuis 1986). These levels were proposed as benchmarks with which to compare seagrasses from polluted nearshore waters from other parts of the archipelago. The Indonesian pulp-and-paper industry is rapidly expanding, which is cause for concern, since pulp-mill effluents contain many highly toxic and ecologically persistent artificial substances such as dioxins and chlorinated aromatic hydrocarbons. These substances have caused serious environmental damage throughout the world, and pose a major threat to coastal ecosystems in the archipelago. The excessive use of mercury in North Sulawesi by the gold mine industry is a cause for serious concern, and proper management of toxic wastes must be implemented.
Oil Pollution. Fortes (1988) stated that seagrasses in Southeast Asia were "generally resistant" to oil pollution. This conclusion was based on the assumption that the sea-grass plant community has a "buffering" effect that will protect the below-ground root/rhizome mat and fauna from direct contact with oil. It was assumed that recovery would proceed from the roots/rhizome mats. It was further assumed that floating oil had no impact on submerged seagrass leaves and the root/rhizome mats. A similar view was held by Phillips (1984), who suggested that seagrass may be less sensitive to oil than their associated infauna and epifauna. An oil spill of at least 50,000 barrels of medium-weight crude oil that spilled into the sea from a ruptured tank on the Caribbean coast of Panama, provided the first opportunity to study the effects of oil spills in shallow-water tropical coastal environments. The study that was conducted immediately following the spill revealed that entire intertidal Thalassia testudinum seagrass meadows were killed by the oil spill (Jackson et al. 1989). The dense seagrass foliage did not provide any buffering capacity to the system, and both the above- and below-ground plant parts were killed. In contrast, subtidal sea-grasses survived, even though they were discoloured, thus largely substantiating an earlier view by Fortes (1988). However, Jackson et al. (1989) were the first to demonstrate that a significant component of the seagrass infauna was also killed. Sloan (1993b) suggested that the most useful approach to address and plan contingencies for possible oil pollution impacts (i.e., oil spills) on seagrasses is on an ecosystem level.
Aquaculture. Brackish-water fish and shrimp pond (tambak) culture is an important Indonesian coastal zone industry. Tambaks are constructed along coastal flatlands by reclaiming mangrove areas and/or closing off areas by dikes. Sloan (1993a) suggested that seagrass meadows can be directly or indirectly affected by tambak development. In instances where tambaks are built on reclaimed mangrove lands, seagrasses frequently suffer from increased siltation due to the loss of the mangrove "filter" which buffers the seagrasses from excessive land runoff. Removal of mangroves will also cause changes in local hydrology and coastal circulation patterns. However, tambak effluents are the main concern, since they contain exceptionally high nutrient concentrations. High nutrient levels in tambak waste-waters are generated mainly by the rapid aerobic decomposition (oxidation) of excess feed material, and by the metabolic processes (i.e., excretion) of millions of shrimps. Phosphate and nitrate-nitrite nitrogen concentrations are high enough to cause localized eutrophication in areas with insufficient flushing. It has also been reported that some operators use pesticides, such as the chlorinated hydrocarbon "Thiodan" to initially kill all trash species before stocking ponds with fry (Giesen et al.1990).
Overfishing. Subsistence harvesting of seagrass-associated organisms occurs throughout Indonesia. In many parts of the archipelago, seagrasses themselves are harvested and used for a variety of purposes (see Fortes 1990 for review). How many of the coastal seagrass ecosystems are overharvested has not been documented. In Banten Bay, local fishermen use beach seines to catch shrimps and small fish (e.g., Stolephoruss pp., known as ten). While the abundance of engraulids (Engraulidae, anchovies) and clupeids (Clupeidae, sprats) has generally kept pace with increasing demand, many larger fish species (e.g., siganids) are becoming locally scarce (e.g., Kepulauan Seribu). Whether this is due to overharvesting or to destruction of seagrass habitats has not been ascertained. In areas with lower population densities, such as Kuta and Gerupuk Bays, south Lombok, seagrass resources are still relatively abundant, and coastal people collect edible fauna (especially the burrowing biota, i.e., Bivalvia) from the seagrass meadows by "gleaning", and by collecting seagrasses during low tides (Kiswara and Winardi 1994). Coastal fishermen use a number of fishing techniques (i.e., nets and/or traps) in coastal seagrass beds to catch fish. One such technique is the kelong (fig. 18.55), a combination of bagan (i.e., lift-net) and sero (i.e., wooded stationary fish trap), placed in seagrass beds (Hutomo et al. 1985; Kiswara and Tomascik 1994).
Geographical Overview of Seagrass Communities
The status of major Indonesian seagrass meadows is assessed on a provincial level. The information has been obtained from a wide variety of sources, including published and unpublished material. The Indonesian seagrasses, however, are the least-studied coastal ecosystems compared to mangroves and coral reefs. There is still no information on seagrass ecosystems from large parts of Sumatra, Kalimantan and Irian Java.
South Sumatra.
LAMPUNG BAY. Seagrass beds at Legundi Island are found on the intertidal reef flats and on sandy reef slopes down to a depth of about 8 m (Kiswara 1991b). The average sea surface temperatures are 29.5°C and 29.3°C to a depth of c. 10 m. Several rivers discharge into the bay, and during the Northwest Monsoon (i.e., rainy season) the bay waters are often laden with sediment derived from land erosion (Prawoto et al. 1992).
The seven seagrass species that are found in Lampung Bay are: Enhalus acoroides, Cymodocea rotundata, C. serrulata, Halophila ovalis, Halodule uninervis, Syringodium isoetifolium and Thalassia hemprichii. Bottom sediments in the bay are composed primarily of coral sand and rubble, with fluvial deposits along shorelines in close proximity to rivers. Depth of substrate varies between 5 to 40 cm. Seagrass communities vary from mixed to monospecific meadows, with cover ranging from 5% to 80% of available substrate. Two types of reef are found in the bay, namely fringing reefs and patch reefs (supratidal, intertidal and subtidal). Reef flats of the fringing reefs are between 22 to 80 m wide. Seagrass beds associated with the reef flats vary considerably in area, but commonly they are between 3 to 60 m wide (Kiswara 1991b).
Southwest Java Sea.
BANTEN BAY. Ban ten Bay is located on the northwest coast of Java. The bay has an area of about 120 km2, and contains several vegetated coral cays. The largest inhabited island is Pulau Panjang. The other islands are small and uninhabited. Several rivers (i.e., Domas, Soge, Kemayung, Banten, Pelabuhan, Wadas, Baros and Ciujung) open and discharge into the bay. The estimated area of seagrass beds in the bay is about 125 ha. Seagrass beds are found from the intertidal down to a depth of about 4 m (Kiswara 1992a).
Figure 18.55. Typical kelongoften seen in coastal seagrass beds. A - hut; B - lift net (bagan); C - fish trap.
From Martosewojo et al. 1983.
The average depth in the bay is less than 10 m, and the sediments consist of mud and sand (Kiswara 1992a). Because of numerous rivers and small streams, salinity fluctuates between 28.1 psu to 35.3 psu (Hutomo 1985). Lowest salinities occur during the rainy season (i.e., Northwest Monsoon) from November through March. Mangrove stands are found from Grenyang (east part of the bay) to Tanjung Pontang (west part of the bay), as well as along the south coast of Pulau Panjang.
Seven species of seagrasses are present in Banten Bay, namely Cymodocea rotundata, C. serrulata, Enhalus acoroides, Halophila ovalis, Halodule uninewis, Syringodium isoetifolium and Thalassia hemprichii. Seagrasses in Banten Bay form both monospecific and mixed meadows. Monospecific meadows are composed primarily of E. acoroides, while the most common mixed seagrass association consists of E. acoroides, C. serrulata, H. uninewis, and T. hemprichii. Seagrass beds are found along the mainland, where they grow on terrigenous sediments, as well as on offshore coral cays (Pulau Panjang, Pulau Tarahan, Pulau Lima, Pulau Kambing and Pulau Pamuyan Besar). Seagrasses associated with patch reefs are found in both intertidal and subtidal habitats. Substrates are usually mud, sand and coral rubble, depending on location. Seagrass cover in the meadows varies from 5% - 80%. Enhalus acoroides is often the dominant seagrass species (Kiswara 1992a).
The seagrass beds support a diverse benthic fauna, with 66 species recorded. The benthic macroinvertebrates in the seagrass beds comprise 28 crustacean species, 25 species of polychaetes, 10 species of molluscs and three species of echinoderms (Aswandy and Hutomo 1988). Hutomo (1985) found 165 fish species, many of them juveniles, and concluded that the seagrass beds act as nursery grounds. Kiswara et al. (1991) collected 85 species of fishes, most of them also in the juvenile stage. Many of the juvenile fish found in the seagrass meadows are commercially valuable species. Juveniles of six species of groupers, namely Epinephelus bleekeri, E. fuscoguttatus, E. mera, E. septemfasciatus, E. tauvina and Plectropoma sp. were recorded in the seagrass beds, with E. tauvina being the most abundant species (Sugama and Eda 1986). Seagrasses are also harvested as feed for a captive dugong in Ancol Aquarium. Hendrokusumo et al. (1979) reported a number of dugong sightings in Banten Bay and suggested that Banten Bay may be an important dugong habitat. However, the last dugong netted by fishermen in 1974 was also the last sighting.
KEPULAUAN SERIBU. The Kepulauan Seribu patch reef complex is located northwest of Jakarta in the southwest Java Sea. Seagrass beds are found throughout the reef complex and are associated with reef flats and lagoonal environments. The most frequently studied meadows are at the Pari Island Complex, which is a multi-lagoonal patch reef with five coral cays (i.e., Pari, Tengah, Kongsi, Burung and Tikus). The total area of the reef complex is about 12 km2.
Sediments in seagrass beds consist of mud, sand and coral rubble (Kiswara 1992b). Sea surface salinities and temperatures fluctuate between 28.5 psu to 35 psu and 27.1 °C to 31.9°C, respectively. Suspended particulate matter concentrations in the water column vary during the year between 1.2 mg.l-1 (Southeast Monsoon) to 14.20 mg.l-1 (Northwest Monsoon) (Nurhayati 1993).
Seagrasses are found on reef flats in the intertidal zone down to a depth of c. 2 m. Eight seagrass species are found in the area, namely Enhalus acoroides, Cymodocea rotundata, C. serrulata, Halodule uninervis, Halophila ovalis, H. minor, Syringodium isoetifolium and Thalassia hemprichii. The dominant species on the intertidal reef flats, which is exposed at every low tide, is Thalassia hemprichii Seagrass cover is low, usually less than 10%. Enhalus acoroides is dominant in sub tidal habitats, where it may form extensive monospecific meadows (Kiswara 1992b). The diverse macroinvertebrate fauna of Kepulauan Seribu seagrass communities comprises 11 species of foraminifera, 29 species of crustaceans, 58 species of polychaetes, five species of echinoderms, 18 species of molluscs and 29 species of phytoplankton collected from the lagoonal waters (Hutomo and Martosewojo 1977; Pratiwi et al. 1993; Suhartati 1993; Thoha 1993).
Sunda Strait.
MISKAM BAY. Miskam Bay is located in the southeast Sunda Strait. Seagrass beds cover an area of about 30 ha, from the intertidal down to a depth of c. 6 m (Kiswara and Tomascik 1994). During the Northwest Monsoon (November-March) Secchi disc depth varied between 0.30 to 1.50 m, reflecting high concentrations of suspended solids. Sediments in seagrass meadows consisted mostly of mud, sand (fine sand and coarse sand) and siliciclastics (Kiswara and Tomascik 1994).
The most extensive seagrass beds were found along the coast between Desa Cipanon and Tanjung Lesung. The seagrass bed is approximately 2.5 km long and between 30 to 250 m wide. The percentage of seagrass cover varied from 5% to more than 50%. Eight seagrass species were collected from the area, namely Cymodocea rotundata, C. serrulata, Enhalus acoroides, Halodule pinifolia, H. uninervis, Halophila ovalis, Syringodium isoetifolium and Thalassia hemprichii (Kiswara and Tomascik 1994).
SOUTH SULAWESI. The Spermonde Archipelago is located along the west coast of South Sulawesi, in the southeast Makassar Strait. The area covered by seagrasses has been estimated to be 240 km2. Seagrass beds are found from the intertidal reef flats down to a depth of c. 30 m (Erftemeijer 1993).
Seagrass beds along the main coast of Sulawesi are found on terrigenous sediments, while seagrass beds on the offshore islands are associated with intertidal reef flats as well as subtidal habitats. Reefal sediments consist of a mixture of coarse, medium and fine carbonate sands. The terrigenous sediment consisted of sandy mud (Erftemeijer 1993). Tidal range is about 120 cm and has a pronounced effect on community structure and zonation. The offshore coral islands are located in clear water with high Secchi-disk readings (c. 14 to 26 m). Salinities were relatively constant, and average about 34 psu. Surface water temperature varies between 26.5°C and 32.5°C.
Seagrass beds were found to occur in four different habitats: 1) offshore reef flats, usually less than 2 m depth at HW; 2) reef slopes at a depth of 10-18 m; 3) shallow bays; and 4) intertidal coastal mud flats. Eleven seagrass species are found in the area, namely Cymodocea rotundata, C. serrulata, Enhalus acoroides, Halodule pinifolia, H. uninervis, Halophila decipiens, H. ovalis, H. minor, Syringodium isoetifolium, Thalassia hemprichii and Thalassodendron ciliatum. T. hemprichii and E. acoroides were the dominant and constant species in the mixed beds on the reef flats. H. uninervis and C. rotundata are considered pioneering species, occupying marginal areas near the beach and exposed sites close to the reef edge, where other species are absent. Thalassia hemprichii, S. isoetifolium and C. serrulata account for more than 75% of the total seagrass cover. Monospecific meadows of Enhalus acoroides occur at Gusung Talang, subjected to frequent river runoff events (Erftemeijer 1993).
SELAYAR ISLAND. Selayar Island is located in the Flores Sea. Seagrass beds are found from the intertidal zone down to a depth of 15-20 m (Nienhuis et al. 1989). The tide is irregular semi-diurnal with a tidal amplitude of approximately 1.5 m. The area is characterized by clear coastal waters, with only a few clear-water streams running into the sea. Sea surface water temperatures during September varied between 25°C and 29°C.
Selayar and the adjacent islands have a number of seagrass habitats, such as silty mangrove bays, sheltered sandy lagoonal environments on the leeside of small islands, and open coastal and slightly inclining inner reef flats that are up to 500 m in width. Eight species of seagrass were found in the area (i.e., Enhalus acoroides, Cymodocea serrulata, C. rotundata, Halophila ovalis, Halodule uninervis, Syringodium isoetifolium, Thalassodendron ciliatum and Thalassia hemprichii). Monospecific and mixed seagrass assemblages were present. Thalassodendron ciliatum meadows were found only in stable subtidal localities, frequently among coral heads. T. ciliatum has been observed rooting in coarse-grained sediments, coral rubble and on massive coral colonies (mostly Porites spp.).
TAKA BONE RATE ATOLL. Taka Bone Rate Atoll is located in the Flores Sea, east of the southern tip of Selayar Island. The atoll has an area of about 222,000 ha and a large lagoon with a depth of about 20-80 m. The atoll is characterized by clear oceanic waters, and the lagoon is filled with extensive lagoonal patch reefs of varying geomorphologies. Large areas of the reef flats are intertidal and are covered with unstable sand and sand banks with only very little seagrass growth. On more stable and slightly inclining reef flats and lagoons, seagrasses are more abundant, forming extensive meadows. The outer reef slope beyond the reef crest descends abruptly to deeper water (Nienhuis et al. 1989).
Six species of seagrasses were recorded on the atoll (i.e., Cymodocea rotundata, Halodule unineruis, Halophila ovalis, Syringodium isoetifolium, Thalassia hemprichii and Thalassodendron ciliatum). Seagrasses form monospecific and mix meadows. Monospecific meadows are frequently dominated by T. hemprichii, while-mix meadows are often monopolized by H. ovalis, H. unineruis, E. acoroides and T. hemprichii associations. Mixed meadows were not found on recently deposited or unstable sediments. T. hemprichii meadows are the most abundant and widespread. Halodule unineruis often forms almost monospecific beds in unstable habitats of the inner reef flat, or on steep sediment slopes (Nienhuis et al. 1989). The green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles were frequently observed, and both nest on uninhabited cays.
A total of 121 species of gastropod molluscs, 78 species of bivalves and one scaphopod (Scaphopoda) were recorded, including nautilus, cuttlefish, squid and octopus. However, despite the high diversity, animal population numbers were generally low, indicating overexploitation. Six of the seven species of giant clams were recorded at Taka Bone Rate, the most abundant being Tridacna crocea and T. maxima.
West Nusa Tenggara.
KUTA AND GERUPUK BAY. Kuta and Gerupuk Bays are located on the south coast of Lombok. Gerupuk Bay is about 80 km south of Mataram. Seagrass beds cover an area of about 250 ha. Seagrass meadows are found from the intertidal zone down to a depth of about 10 m. The sediments consist of mud, fine sand, sand, coarse sand, coral rubble and dead coral (massive). The tidal range is about 3 m (Kiswara and Winardi 1994).
Eleven species of seagrass were found at these two locations, namely Cymodocea rotundata, C. serrulata, Enhalus acoroides, Halodule pinifolia, H. unineruis, Halophila ovalis, H. minor, H. spinulosa, Syringodium isoetifolium, Thalassia hemprichii and Thalassodendron ciliatum. Seagrasses formed monospecific and mix meadows. The seagrass cover varied from 5% to 100%. Enhalus acoroides was the most abundant species (Kiswara and Winardi 1994).
The seagrass-associated fauna was represented by: 1) 70 species of crustaceans belonging to 16 families and 50 genera (Moosa and Aswandy 1994); 2) 70 species of molluscs belonging to three classes (Gastropoda, Bivalvia and Cephalopoda) (Mudjiono and Sudjoko 1994); and 3) 45 species of echinoderms from five classes and 28 genera (Azis and Soegiarto 1994). Meiofauna was dominated by nematodes, foraminiferans, copepods, ostracods, turbellarians, and polychaetes (Susetiono 1994). A diverse fish fauna was composed of 85 species belonging to 47 families and 60 genera (Hutomo and Parino 1994). Sea turtles nest on the beach from November to April.
East Nusa Tenggara.
KOMODO ISLAND. Komodo is located about 30 km off the west coast of Flores in the Sape Straits which separate Flores and Sumbawa. The area of marine waters is about 10,000-12,000 ha; depth varies between 15-20 m; the highest peak is Gunung Satalibo at 735 m.
Coastal waters around Komodo Island are clear oceanic with very little terrigenous influence. The coastline is dominated by rugged, rocky shores with steep dropoffs into the sea. Beneath the water the slope continues down to a depth of 1520 m, where it usually flattens out into a sandy bottom.
Seagrasses were found in habitats ranging from sheltered localities fringed by mangroves and dominated by silty to fine-grained sediments with coral colonies, to more exposed localities with sandy beaches and slightly inclining sand- and rubble-covered reef flats. Seven species were found in the area, namely Enhalus acoroides, Cymodocea rotundata, C. serrulata, Halodule unineruis, Halophila ovalis, Syringodium isoetifolium and Thalassia hemprichii. Both monospecific and mixed meadows were found in the area. The mixed seagrass beds did not occur in extremely sheltered, silty habitats near mangroves, or in the upper part of the intertidal zone where desiccation prevails during the low tides. Thalassia hemprichii may also form monospecific stands. The vegetation has a large vertical range from the intertidal zone down to the lower sub tidal zone, where T. ciliatum dominates.
Enhalus acoroides meadows are widespread, especially in silty sediments, but it was observed to root in medium- to coarse-grained sediments as well. For example, the inner reef flat of the fringing reef that runs along most of the north coast supports dense meadows of E. acoroides as well as mixed meadows composed of E. acoroides, H. ovalis and T. hemprichii. E. acoroides also forms extensive monospecific stands in silty subtidal habitats, or localities with heavy bioturbation where E. acoroides survives because of its firm anchorage, provided by large rhizomes (Nienhuis et al. 1989).
Moluccas: Kotania Bay. Kotania Bay, with an average depth of c. 30 m, is located on the west coast of Seram Island (Wouthuyzen and Sapulete 1994). Seagrass meadows in shallow-water coastal areas cover an area of about 1115 ha. Sea surface salinities fluctuate between 21.8-34.7 psu, while sea surface temperatures range between 27.5°-29.9°C. Coastal circulation patterns are dominated by tidal forces. Average current velocities during flood and ebb tides are 0.07 m.sec-1 and 0.11 m.sec-1, respectively (Hutahaean 1992).
Seagrass beds grow in shallow intertidal areas including sand bars and coral reef flats. Four seagrasses have been recorded from this area, namely Enhalus acoroides, Halophila ovalis, Syringodium isoetifolium and Thalassia hemprichii. T. hemprichii is the most abundant species at Osi Island, Burung Island and south of the Lalansoi area. Rahayu (1984) recorded 60 species of seaweeds from three divisions: Rhodophyta (red algae) with 26 species; Chlorophyta (green algae) with 24 species, and Phaeophyta (brown algae) with 10 species.
A total of 207 species offish belonging to 52 families were collected in the area (Osi and Marsgu Islands). The dominant families are Apogonidae (9 species), Chaetodontidae (8 species), Lethrinidae (8 species), Pomacentridae (21 species), Labridae (14 species), Siganidae (15 species), and Tetraodontidae (7 species) (Peristiwady 1994a). Rahayu (1994) found 63 hermit crabs species belonging to the Families Diogenidae and Paguridae.
Associated with the extensive reef flat seagrass beds are economically important seaweeds, such as Gracillaria spp., with an average biomass of about 994 g.m-2. Since the total area covered by Gracillaria is about 40 ha, the total biomass of the algae is roughly 398 tonnes. Another economically important seaweed is Hypnea spp., with an average biomass of 41 g.m-2, which translates into 16.5 tonnes for the area (Rahayu 1984).