9
It is not clear whether an argument can be made that birds moved from living in loose aggregations of individuals to pair bonds and to complex negotiated relationships in cooperative contexts over evolutionary time (Shultz and Dunbar 2007). This is difficult to establish, particularly because all forms of aggregation exist in currently living birds. However, the majority of Australian birds live in some form of partnership and, more than elsewhere perhaps, in Australian birds such bonds tend to be lengthy, involved and often permanent. Any of these conditions are said to foster the evolution of a larger brain, at least according to the social brain hypothesis.
The social brain hypothesis, as was already alluded to in the Preface, argues that large brains have arisen in response to the social dependence and ecological conflicts and, in such contexts, decision making has been thought to be of particular importance (Dunbar 1998). Such theories when applicable to animals were largely made with primates, and foremost chimpanzees, in mind (Shultz and Dunbar 2007). In 2012, McNally and colleagues showed that selection for efficient decision making in cooperative dilemmas can give rise to selection pressures for greater cognitive abilities, and that intelligent strategies can themselves select for greater intelligence (McNally et al. 2012).
Against this view Barrett and Henzi (2005) have argued that the social brain hypothesis is seriously flawed because it is circular: ‘Primates have large brains because their social lives are cognitively demanding, and their lives are cognitively demanding because they have large brains that allow them to produce more complex forms of social behaviour.’
Instead of large theory building, some scholars have chosen to stay with measurable small units and see what birds can actually hear, see, smell, and feel and what we know of these sensory inputs. There are good reasons to do so. As it turned out, the greatest expansion of the primate brain over evolutionary time occurred in the visual cortex (in particular, area V1) and in the parvocellular region, which is associated with analysis of fine detail and colour. Barton (1998) and later Barrett and Henzi (2005) explained that it was not so much that primates needed to see or hear more in the environment but, as they formed larger and socially more cohesive groups, their perceptual system needed to be enhanced in order to process details of dynamic social stimuli, such as facial expression, posture, gaze direction and the like (Barton 1998).
Whether this argument holds true for birds is an open question. However, when we examined the brains of Australian magpies for song nuclei (Deng et al. 2001), we also found that the area in the brain called the Wulst – an associative cluster that is known to be involved in coordinating visual and even spatial and motion information – is a very large and clearly identifiable region in the brain. The avian visual Wulst is in some ways equivalent to the mammalian primary visual cortex, called V1 (Karten et al. 1973). Owls have a particularly massive visual Wulst, which apparently shares striking functional similarities with V1 in humans (Pinto and Baron 2009). And, indeed, the eyes of birds are marvels among the eyes of vertebrates. The avian retina not only has one of the most sophisticated cone photoreceptor systems (with five types of cones), these systems are integrated on the retina in five regularly spaced, but independent, ‘tile’ systems: a critical precondition for uniform sampling of visual space and accuracy of spatial sampling of the world (Kram et al. 2010). The authors who recently published these findings suggest that this organisation of the cones of the retina (i.e. the colour vision) is common to all birds.
In other words, birds may see more now than some million years ago but this perceptual improvement could have been for better food detection and generally for better discrimination of partly occluded things or, indeed, it might have been for paying close attention to their peers, or a mixture of all these facets. Uniform sampling of visual space and accuracy of spatial sampling of the environment would certainly lead to more acute assessment of the environment, but that could also include peers.
A movement away from loose aggregations of animals, as in herds of ungulates or colonial nesting or flocking in birds, to close associations might not require changes in song nuclei but in powers of perception: a watchful eye, power of observation and careful scrutiny of others for any signs of aggression, danger or feeding advantage. Watching others means awareness of others and such habits can change from behaviour reading into mind reading.
Signals by individuals may be given unintentionally and spontaneously but it may be important for the receiver of such signals to be aware of what they might mean. For instance, understanding rising anger can avoid an attack and so can the flagging of an affiliative and friendly approach. Recognising individual features and responding to close encounters may also be an advantage. The harder part for testing is to relate both (sender and receiver) and it is even harder to ask whether the signal was intentional, whether it revealed or communicated thoughts or emotions and how such emotions may be appeased if agonistic or find support if fearful. It was Charles Darwin within his enquiries into evolution who first braved the subject of emotions in animals (Darwin 1965) but, despite his book on the subject, it took another 100 years before emotions were placed on the agenda (cf. Summary Kaplan and Rogers 2004b).
Study of emotions
Emotions are a difficult subject. As Tinbergen observed, animals do basically four things: feed, fight, flee and fornicate. These can be described and the ‘how’ and ‘what’ of such actions can be measured (e.g. by Tinbergen in gulls). Tinbergen also said that these four ‘fs’ of bird actions sprung from motivational systems present in all animals and basic to survival: hunger, aggression, fear, and sex (see Table 9.1).
These categories have been related to physiological processes underlying the different types of behaviour. In 1953, Tinbergen argued that behaviour was due to relatively invariant and immediate responses to internal and external stimuli (Tinbergen 1953). This was a starting point, and a very important one, but research into emotions in animals generally, and birds specifically, has since moved ahead substantially. Indeed, the debates about methods of studying emotions are currently heated and ongoing.
Table 9.1. Shifts in studying emotions in animals in the last 50 years of research in animal behaviour, in a range of vertebrate species including birds.
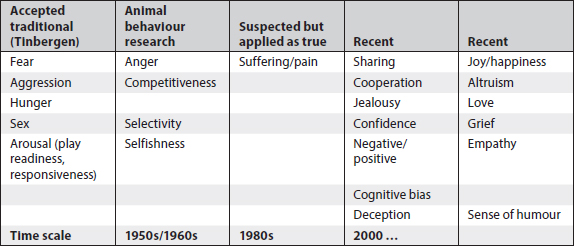
Fear was probably the first emotion systematically investigated and, in most cases, fear is related to recognition and memory of something unpleasant or dangerous, in any modality. The growl of a panther can be as spine chilling as seeing one and survival often depends on this fear, catapulting the individual into fast actions. While this sounds simple, and fear triggers responses very swiftly – enabling escape for instance – why and how such emotion developed and how it is processed has been a matter for debate for a long time.
It seems entirely inconsistent to consider emotions in the context of a broader exploration of cognitive behaviour in birds, but the two are not so far removed in the processing of them in the brain. Evidence for this has come from studies of brain lateralisation. MacNeilage and colleagues recently summarised the origins and functions of the left and right brain. They said that the division of labour by the two hemispheres dates back half a billion years, although it was once thought to be unique to humans (MacNeilage et al. 2009). Speech, facial recognition and the processing of spatial relations has now been traced back as far as early vertebrates (i.e. preceding the evolution of birds). Vocal performance in birds, as in humans, is controlled by the left hemisphere and so is discrimination of objects (food from non-food) while the right hemisphere is responsible for intense emotions (such as fear of attack) and these are largely controlled by the amygdala: a structure just beneath the forebrain. Hence, the right hemisphere expresses intense emotions and the left hemisphere may inhibit some of these strong responses (Rogers et al. 2013).
That face recognition has an early evolutionary origin is important here and it is a function of the right hemisphere, as shown in several species. There are now several studies showing that some birds also learn to recognise human faces (Barnett et al. 2013; Bogale et al. 2011a; Kondo and Izawa 2014) and will particularly remember those that are perceived as threatening and dangerous (Cornell et al. 2012; Lee et al. 2011; Marzluff et al. 2010).
Experiments with chicks have provided strong evidence that birds recognise faces of conspecifics. In a very nice experiment, domestic chicks were tested singly in a runway from where two chicks could be seen through transparent barriers at either end, one on the left and one on the right. On one side was a stranger and on the other a cage mate. If the left eye was occluded, meaning that the left hemisphere received the visual input, the chick did not discriminate between the stranger and the cage mate and spent about equal time with each. When the right eye was occluded (just a patch over the eye), so the right hemisphere received the visual input, the chicken went directly to its cage mate and did so without hesitation and then spent most of its time with its cage mate (Vallortigara and Andrew 1994). This is because the right hemisphere can process details, but the left hemisphere can only process categories: that is, it responds to other chicks as a species but does not take into account the differences between individual chicks. I shall come back to the importance of the ‘face’ for close, and even intimate, communication but first it is important to outline how emotions can be studied methodologically.
Methods of studying emotions
One of the methods used in great ape studies was to teach apes sign language. This was an odd choice because it presumed that animals without a language system like that of humans would nevertheless be able to express themselves in a symbolic translation of human language. Yet the method worked and apes learned to express or give a vocabulary or symbol to many objects and people. They also expressed emotions via sign language. There was the famous example of Koko, the gorilla, who expressed sadness when thinking about his friend, the kitten, that had died some years earlier, suggesting that the gorilla felt grief and sorrow (Patterson and Linden 1981). In birds, the method of trying to communicate via language or symbols with a degree of reciprocity has only been tried systematically with one bird: Alex, the African grey parrot (Pepperberg 1987).
In neuroscience, techniques have now been found to test brain activity in a living bird (a corvid) by first injecting it with labelled glucose, then testing the bird on a behavioural task followed by mildly anaesthetising the bird to produce PET scan of its brain. It showed the areas of the brain that were active when the bird was actively looking at the masks worn by humans. They found that the right hemisphere amygdala was active in response to seeing a feared face but the left hemisphere was active when a food-associated face was presented and neither responded to a neutral face (Marzluff et al. 2012). This is such a convincing outcome bearing in mind that, behaviourally, very little could be observed. No doubt, this new technique (i.e. keeping the bird alive), will be applied more widely in future and yield valuable results, not just about cognitive processes but also about states of emotions.
Stress and pain have been measured using particular methods, but even in this category marked changes have occurred. An older and familiar way of testing negative emotions is via established sampling methods in physiology. Blood, urine or faecal samples were collected and from these samples assays were prepared showing, for example, the level of stress hormones in a particular animal (the stress hormone in mammals is cortisol, whereas the main stress hormone in birds is corticosterone). The old method, especially blood sampling, had itself a stressful effect and could thus distort the results. To limit or eliminate this variable, we devised a new method testing whether taking saliva samples from primates could not yield results, and it did. In our laboratory, we did this using cotton buds that had been smeared with their favourite food (banana). The primates (marmosets, a small South American primate species) relished the treat and readily volunteered for the task, even helping to hold the bud (Cross et al. 2004). The saliva was then assayed for stress hormones. We were thus able to take regular samples and able to record the effects of unpredictable events and on hormonal levels.
In birds, a relatively non-stressful and less invasive way of measuring stress hormones than blood sampling is by taking a sample of feathers (Bortolotti et al. 2008, 2009). Assays from feathers have been shown to measure the stress hormone corticosterone effectively and the preferred method is not to cut the feather by holding the bird down but by food enticements, a pair of tweezers and a quick extraction of the downier breast feathers. Again, this recent method can provide a good deal of information especially when the method seeks to distract the bird by delivering a reward during extraction of a feather.
Laboratories are usually not set up for unpredictable events and hence some of the reactions to extraordinary events are missed. When we conducted behavioural experiments with marmosets, the project required daily sampling of cortisol. Changes in cortisol levels were thus recorded and capable of being monitored on a regular basis. Any irregular or unplanned events could therefore also be read against the readings of cortisol levels. Obviously unexpected was a fatal accident suffered by one marmoset (falling off a branch after a jump and breaking its spine) during the experimental period, but outside any active trials. We found that in the surviving and related roommates cortisol levels had risen substantially. Within a few hours of the accident, cortisol levels in onlookers rose and continued rising. In the afternoon, when cortisol levels in marmosets normally decline, levels stayed up and remained high for 3 days (Kaplan et al. 2012).
There are two reasons why this experience with a primate is related here. First, the very fact that stress levels revealed a strong emotional response in a survivor is remarkable. We never credited animals with such feelings of distress on behalf of another animal. Seeing the other fall and then responding physiologically in such a powerful way perhaps suggests empathy and certainly fear at the time. It is also puzzling that the effect lasted for 3 days (without any exposure to the dead marmoset), suggesting trauma or even grieving.
The second reason is an even more important lesson for the study of emotion. Had we not incidentally measured cortisol levels, we would not have known that the marmoset was affected at all. Any outward signs of stress in this primate were minimal. While the marmoset shrieked (‘tsik’ calls) at the time of the fall, there was little else to observe thereafter and nothing to indicate that the loss of the cage mate actually had such a profound effect on the survivor.
Our ability to detect pain or stress in birds is even more difficult than in a primate or a dog. Once a boobook owl was delivered to my door. One wing was almost entirely severed and barbed wire had been embedded in part of its chest. The bird was bleeding but conscious. The driver who brought the bird said it seemed to have a ‘bit of an injury’ but otherwise it was ‘happy’. While this is not the first thought that would have come to my mind when a bird presents with half a wing hanging off its body, the driver was only responding to expectations of what we know might happen in such a case when a human is severely injured. However, in the owl’s behaviour any indices of suffering were missing: there was no trembling, no screaming or moaning. The only indication of pain, as I have found in many years of looking after birds, injured or healthy, is that the eyelid may quiver a little or the eyes remain half-closed and, occasionally, the body posture may be a little hunched.
Pain and suffering have been difficult to assess in animals (Bateson 1991). Assessing pain reliably and species by species (because there may be differences) might literally require torturing animals and this is not acceptable. The problem with pain in animals is that any form of outward expression of pain may be missing (Dawkins 1980). There may be no sounds, no contortions or other clear behavioural indices to suggest the presence of pain. This is an important adaptation in the wild, and particularly well developed in birds, and is called pain masking. There are feelings and experiences that birds do not want to communicate and advertise to avoid attracting attention by predators. That does not mean that the bird experiences no pain. In my own experience, while not entirely compelling evidence, it is clear that birds that I have treated with painkillers after trauma (fractures/flesh wounds) recovered twice as fast as those without such assistance and many more survived extreme trauma that might otherwise have been fatal. While this is only soft evidence, it is sufficiently convincing to continue on this course of action.
Still, decades of meticulous research have generated reliable data on the circumstances fostering the occurrence of stress, as well as the mechanisms that reduce stress. The literature on this aspect of animal behaviour is now vast and well beyond the scope of this book. Suffice it to say that stress levels are lower in birds surrounded by familiar individuals, lower in species with larger brains and, in case of fearful contexts, stress hormone levels return to normal more quickly in species with larger brains. Allogrooming is a way of reducing tension within a group and play behaviour decreases stress and leads to better health and mental function.
The second methodological approach to studying emotions in birds has come from cognitive science using purely behavioural experimental methods. At first, the method was tried in humans, then applied to rats and published in Nature in 2004 (Harding et al. 2004). It introduced the idea of cognitive bias into performance on ambiguous stimuli, positioned between two opposing outcomes and experiences (gained via training the animals): one negative and one positive. The ones taken from drab (i.e. negative) environments were asked to respond to a set of ambiguous stimuli that might or might not hide a delectable food item or could be empty or dangerous and the same procedure was repeated with subjects taken from enriched environments.
Melissa Bateson and her colleagues at Newcastle University UK used this paradigm to test captive and tame starlings, starting off with the assumption – as Marian Dawkins had done in her chicken experiments many years before (Dawkins 2000) – that one can ask important welfare questions by asking the birds themselves. They investigated how starlings respond to different living conditions by giving them choices. The method is surprisingly simple: house the birds in different environments, train the birds to open lids of white boxes and the box will invariably contain a treat (Bateson and Matheson 2007). Open a box that is dark grey or black, and the box will be empty (or, as a variation, it could also hold an obnoxious item). Ambiguous boxes were several shades of grey from very light to dark. The less often the lid was opened in the ambiguous colouring of a box, the stronger the negative cognitive bias and the more often they were chosen, the more positive the bias.
One experiment was designed to assess whether their outlook was ‘pessimistic’ or ‘optimistic’. Birds were trained to associate a tasty snack – a worm – with a dish with a white lid, and an unpalatable quinine-flavoured worm with a dish with a dark grey lid. Starlings had no problems removing the lids (as other species can also do easily, as discussed in Chapter 7 describing wild blue tits that removed bottle tops) and they quickly learned not to open dark grey lids. The birds were then put either in ‘enriched’ cages with branches and water baths, designed to promote greater welfare, or in standard cages that were small and bare. Then the birds were given dishes with lids of various intermediate shades of grey. The colour gradients created ambiguity as to whether and which shades represented the good treats or the bad ones.
The researchers found a clear correlation between the negative environment and the refraining from opening the lid of the shaded boxes and, conversely, between the enriched environment and the greater number of accessing the ambiguous boxes graded in various shades of grey. Those in bare cages flipped significantly fewer grey shaded lids than those in enriched housing (Bateson and Matheson 2007). In another experiment, the researchers asked starlings to discriminate between light signals that indicated either an instant or a delayed food reward, and act upon them accordingly, again housed in barren or in enriched cages. Those in large and enriched cages were more ‘optimistic’ by opening more lids than the others (Matheson et al. 2008).
The research made headlines in The New Scientist because it really looked like a breakthrough (Hooper 2007) because the researchers had applied robust experimental methods to a problem that had accompanied the study of animals for a long time: namely that one must not infer (guess) an animal’s emotional state and there seemed no way to test it. Now it was shown in a bird that internal states and emotional processes in animals can be experimentally tested by using a cognitive approach and placing emotions squarely in the cognitive domain (Paul et al. 2005).
Indeed, linking emotions and cognition has been studied with great vigour in humans by the cognitive science and pharmacological industry. In animals, the last 10 years have brought many innovative studies and thoughtful critique (Mendl et al. 2009), but so far there are relatively few papers that directly consider the interface between emotions and cognitive processes in birds. In this internationally innovative, even scientifically creative, environment one hopes that more such studies will be published.
The simplest and least sophisticated, but absolutely necessary, first steps in any discussion on emotions are the basic questions: do birds have emotions and how do they manifest themselves? If the first question is tentatively answered as ‘yes’, then the second step is determined by one’s own discipline – whether to look for brain activity, say, of neurotransmitters, or whether to look at the behaviour. It does not really matter at which end one starts such investigations, as long as the results inform each other. The ethologist would then proceed to note anything that appears like an emotional response and also identify the context in which it appears without pre-empting what it might signify. The traditional ethological method of pen and paper has not lost its validity, nor has a camera or camcorder to record any brief instances of emotional expressions. Having done so over quite a number of years, looking at expression of emotions in native Australian birds, I can give some examples here, based now on robust physiological evidence already provided, that such expression is possible. In all instances, the context and/or the responses by conspecifics at least suggested what the action or change of mood might have meant.
Emotions and the ‘face’
The idea that birds have ‘facial expressions’ is quite foreign to many people and there has been little systematic work done on this aspect of avian communication or self-expression, despite some early writing in this field. Andrew (1972), for instance, was the first to point to the autonomic responses in displays. He argued that the initially autonomic response of lowering and raising feathers for the purpose of temperature control could be extended and adapted for displays. That also applies to whole body postures.
As late as 2013, the idea of facial expressions in birds was only politely conceded. For instance, Waller and Micheletta (2013) said:
There are a few compelling examples that could be considered to be facial expressions (in a sense). The facial components of birds’ heads are not particularly mobile and they do not have facial muscles (Waller and Micheletta 2013).
They state this with reference to an anatomical study by Diogo et al. (2008), which examined the evolution of head and neck musculature. In short, so they say in passing, birds have no facial muscles and hence they are largely precluded from further consideration. To be fair, these researchers looked at major musculature of the body including the face. However, in 2003, Homberger and de Silva published a paper solving the riddle of how birds can move feathers on their head seemingly without any musculature (Figs 9.1 and 9.2). They showed that there are mechanical forces in the feather-bearing skin (called the integument) and described in detail the biomechanics of an integumentary musculature in birds (Homberger and de Silva 2003).
Facial expression is achieved by movements of the beak, by independent positioning of feathers below or above the beak, on the ear coverts, on top of the head (the crown), at the nape of the neck and, in some species, also by moving the feathers above or below the eyes independently of other feathers (see also Fig. 9.14).
Birds have open mouth displays or, rather open beak displays, which, together with other body signals, are used in fear or threat displays. Tawny frogmouths use a variety of open beak displays and the inside lining of the large oral cavity is a striking light green colour effectively displaying the enormous size of the beak and making it look more ominous than it actually is.
As a threat, a number of bird species open their beaks, even without vocalisation but sometimes associated with hissing or breathing sounds. Galahs and sulphur-crested cockatoos use hiss sounds together with open beaks, very similar in sound and appearance as in blue tongue lizards. The barn owl, for instance – a bird that is rarely heard – emits an exhaling sound in warning while the beak is half open and then sharply claps the beak several times, often without the slightest change in body posture or feather position.

Fig. 9.1. Diagrammatic transverse section through the feather-bearing integument of the wild turkey Meleagris gallopavo at about mid-length of the neck. (1) epidermis; (2) dermis; (3) rachis of a feather (cut); (4) calamus of a feather; (5) feather follicle; (6) feather papilla; (7) smooth erector feather muscle; (8) smooth depressor feather muscle; (9) smooth apterial muscle; (10) elastic epimysium of the apterial and feather muscles; (11) lamina elastica; (12) fascia superficialis; (13) striated subcutaneous muscle (m. constrictor colli); (14) pars pennae of the striated subcutaneous muscle; (15) collagenous epimysium of the striated subcutaneous muscles, I–III numbered feathers. (Reproduced with permission from Homberger and de Silva 2003.)
In galahs and crested cockatoos, movement of head feathers is very easy to detect, even from some distance. The crest goes up not just in alarm but in states of friendly arousal, in play readiness and in affiliative gestures (Fig. 9.3, see also Fig. 9.11). The feathers that flank the beak (ear coverts) can be ruffled to express anger and possible readiness for attack. Lowering or flattening of feathers is usually associated with fear, but this commonly involves the whole body rather than just the head.
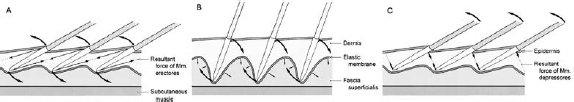
Fig. 9.2. The subcutaneous hydraulic skeletal system: two-dimensional model of the movement of feathers. Cranial is to the left of the diagram, caudal to the right. (A) Feathers in resting position being raised by contracting erector muscles. (B) Raised feathers being returned to their resting position by the resilience of the stretched elastic membrane. (C) Feathers being stabilised against lifting external forces by contracting depressor feather muscles. (Reproduced with permission from Homberger and de Silva 2003).

Fig. 9.3. Play face. (Left) Male galah; (right) male sulphur-crested cockatoo. The galah is 75 years old and the sulphur-crested cockatoo 45 years old and both still quite readily played. In galah crests, each row of feathers can be moved separately and the relative position signals very different things. In a play face, the front row of the crest is up vertically but the back half of the crest is in half-mast position. Note also that both species put out their wings by a fraction. In sulphur-crested cockatoos, play mood is also accompanied by fluffed up feathers around the lower mandible, a display feature largely lacking in galahs. (Note: the two photos are not in scale with each other – the galah is smaller than the sulphur-crested cockatoo.)
‘Cuddly’ and babyish behaviour is often shown by fluffing feathers above and below the beak (readily observable in sulphur-crested cockatoos and galahs as in Fig. 9.3). For close, conspecific interactions, these facial expressions are powerful signals emitted with a minimum of energy expenditure. In most cases, such signals are effective only in intimate situations. Although the change in feather position may simply be an expression of emotions and not expressed intentionally, it is up to the receiver to interpret them correctly or face the consequences.
In addition, like mammals, birds have a wide range of body postures available. Feathers can be moved for purely physiological reasons, of course, such as temperature control, keeping out cold or rain, aerating for better circulation (Andrew 1972) or health care such as anting, dust bathing or skin exposure to sun. Many birds fluff their feathers in a certain way when they are ill, but they may also raise their feathers in different parts of the head or body for a whole host of reasons that may need to be communicated to close partners or group members.
Body postures and feather positions can also be used for signalling a particular message by visual means. Head bobbing, arching of the neck, extending the wings outwards, certain sorts of running, stomping and crouching postures may be used in specific contexts. Quite a variety of body postures are effective signals that may add to facial expression (reinforcing the message) or feather position on parts of the body may indicate a specific state of mind or act as a signal without the involvement of the head at all. Most birds can raise all their body feathers simultaneously, or at least those on their head, to make them look menacingly larger than they are. Such displays are not necessarily accompanied by a vocalisation.
It is possible, and even likely, though, that non-songbirds (non-passerines), comprising many orders and including all parrots and cockatoos, kingfishers, nightjars and birds of prey discussed throughout this book, may have developed more visual signals (feather positions, body postures) than songbirds that tend to specialise in vocal signals. Songbirds in turn may have developed more body postures than non-passerines, using tail and wings for ritual and non-ritualised non-vocal signals and some of them are multimodal, using feather or body signals in conjunction with vocal signals, together creating a rich range of potential signals and communication options that have barely been explored in birds.
Eyes, eye movement and eyelids may at times also contain micro-signals that can be informative either about the state of health of a bird or a particular affect (Zeigler and Bischoff 1993). The tawny frogmouth, for instance, can change the colour of its iris from a greenish yellow colour, when it is in a relaxed state, to a reddish colour when the bird is aroused, perhaps distressed or angry (see image in Kaplan 2007a, p. 91). The red colour is strongest around the edges of the iris and may be caused by dilating the artery, known as the circulus arteriosus iridicus, which circles the iris in this outer region (Martin 1985). With the exception of owls and eagles, birds are able to move each eye independently. Tawny frogmouths can move their eyes in opposite directions and most of their rapid eye movements (known as saccades) are like this (Wallman and Pettigrew 1985). They can also make frequent convergent saccades that turn their eyes inwards to look in front. In humans, saccades have been used as a clue for analysis in psychiatric treatment and in visuo-spatial research (Leigh and Kennard 2004) because they may not just indicate specific eye direction but also suggest affect or stress. There is emerging behavioural evidence that saccades may be coupled with hand movement and gestures, such as an intention to increase grasp precision (Grafton 2009) or pointing behaviour and cognitive gestures made to others. Changes in pupil size may not always refer to light intensity. In tawny frogmouths, after longstanding and close affiliation for research and rescue, I noticed that in males the pupils may become suddenly enlarged (only after dusk) and this change in pupil size is associated with a stare at the female, after which mating follows very quickly. Hence, as in humans, dilation of pupils may be a sexual signal (Laeng and Falkenberg 2007).
Finally, the eyelids may play a role. Eye squinting in a bird that may otherwise sit entirely still may be an indication of pain or irritation, half closing the eyes (i.e. lower and upper eyelid) may indicate the same or general illness. Even the nictitating membrane (sometimes called the third eyelid) can become part of facial communication in the sense that the rate of blinking corresponds to social signalling. This was shown in research on emotional responses by common ravens to faces they had learned to fear (Marzluff et al. 2010; Cross et al. 2013). Given this current state of knowledge, it should perhaps not surprise that, even in birds, the eyes alone may communicate a range of things very effectively.
Agonistic and affiliative signals
One would think that conflict may be as much part of daily life in group-living birds as is cooperation. Too much conflict, however, could divert important energy and, on a regular basis, could endanger the entire group. Any animals that fight with each other are distracted from monitoring their surroundings and at risk of becoming prey items.

Fig. 9.4. Crouching in submission to avoid conflict or punishment within a group. (A) Crouching in a flightless juvenile Tasmania native hen (redrawn from Ridpath 1972). (B) A first-year Australian magpie crouching for the same reasons. (C) Dog (Rhodesian ridgeback) crouching for comparison (not to scale).
How do birds handle conflict among each other, what are the mechanisms to overcome antagonisms and how are such conflicts resolved? These are very interesting questions but we have relatively few studies on any of these aspects in bird behaviour. Researchers have been interested in finding regulatory mechanisms that make peaceful cohabitation and degrees of mutual support possible. There is a study on appeasement in common ravens (Heinrich and Marzluff 1993) and another among Tasmanian native hens (Ridpath 1972). From these studies it appears that birds that spend a good deal of time on the ground foraging – as do ravens, the flightless Tasmanian native hen and also magpies and others – have evolved postures for appeasement that are not unlike those of mammals, such as that shown in a crouching dog, first drawn by Charles Darwin in 1872 (Fig. 9.4).
One study has also looked at the aftermath of conflict for the one perceived to have lost in the conflict. While in certain circumstances it may be too late for appeasement, there is room for consoling the vanquished, at least in human society. In primate society, post-conflict behaviour is most often expressed in allo-grooming. In birds it was not even known to exist. A recent study of raven post-conflict behaviour shed light on this (Fraser and Bugnyar 2010b, 2011). Their study concluded by saying that bystanders may console victims when they share a valuable relationship with the victim thus alleviating the victims’ post-conflict distress. Conversely, the victim may approach the bystander after a conflict in order to reduce the likelihood of renewed aggression.
Anecdotally, I can add here that Australian magpies may well have a similar system of consolation. There is a behaviour that I have observed on several occasions, but in different years and locations. Post-conflict, one could see a magpie standing with hunched up wings in a little spatial isolation from the others (presumably the victim). Another magpie in deliberate locomotion then went over to the isolated magpie and then either just stood next to or touched the victim, extending its neck over the back of the neck of the vanquished individual. After a minute or so in this posture, they would untangle, the hunched magpie would resume its normal posture and walk back to the group together with his/her visitor and resume foraging.
Conflict in groups of psittacine species may concern status issues, resource divisions and small squabbles over space. The question is whether there are differences between species that live in small tight-knit groups versus those living in larger gregarious groups. A study by Pidgeon (1981) hypothesised that fighting in large groups should be higher than in smaller groups, unless means were found to reduce agonistic interactions. A way to study this hypothesis was to investigate species that are gregarious and those that form smaller groups or live in pairs but are phylogenetically relatively closely related and then measure the number of their agonistic vocal and visual signals and compare them with each other. Pidgeon (1981) chose budgerigars, cockatiels, red-rumped parrots, eastern rosellas and galahs.
The fact that Pidgeon (1981) found more agonistic signals in tight-knit small groups than in gregarious species was opposite to his predictions and presented a bit of a puzzle. It might suggest that family groups have more means to communicate their feelings. In sulphur-crested cockatoos and in Australian magpies, both of which form small and tight-knit groups, hierarchical rules are part of their social organisation and these are enforced via an established system of reward and punishment that may partially replace expressions of emotions. For example, as described elsewhere (Kaplan 2008), a juvenile magpie had intercepted the adult male seen feeding. The adult expressed no outwardly agonistic displays but he acted immediately, walking over to the juvenile and administering a swift peck on the head of the juvenile as punishment. The juvenile, just as swiftly, rolled over on its back in a submissive posture, very similar to the posture seen in dogs. Similar hierarchical systems have been studied in detail in wolves and other canine species (Rogers and Kaplan 2001). In sulphur-crested cockatoos, the male seems to literally rule the roost and note is taken by every member to avoid conflict while, in larger groups with less structure, it might be the case that indifference, rather than tolerance, reduces the number of emotional responses.
In all situations, one would imagine, the signals would need to be clear and unambiguous and they ought to make low energy demands. Facial expressions and body postures, as shown below (Figs 9.5–9.11 indicate a range of such close-up ways of communicating with siblings, partners or even strangers at close range, all of which are indeed low in energy demands.

Fig. 9.5. Fear expression. (Left) A stilt’s half open beak and, characteristically, in fear the tongue (clearly visible here) tends to move up towards the upper mandible. (Right) A tawny frogmouth displays mild alarm and shows off the pale green inner lining of the lower mandible. Note also that the eyes are wide open and further gape-widening in conjunction with wide-open eyes indicates threat. Note the slight constriction of the pupil. Both song- and non-songbirds have semi-open beak expressions in fear. A fully open beak tends to indicate threat and, as in the tawny frogmouth and the ostrich, is accompanied by loud vocal noises. (For a full description of the many varied facial expressions in tawny frogmouths see Kaplan 2007a.)

Fig. 9.6. Anger in cockatoos. Both, the galah (left) and the corella (right) can raise feathers around their eyes and on their entire head and neck in expressions of anger. This is not aggression because it is not necessarily followed by attack unless the bird is approached more closely.

Fig. 9.7. Anger in songbirds. Most songbirds have sleek and short feathers around the body and yet, most of them are able to give off micro signals by changing their feather positions either at the nape of the neck or in some specialised position on the head or face. Note the raised feathers forming a hump at the nape of the neck in the scarlet honeyeater on the left. This would normally be a sleek and slender line. The two images on the right of an adult male Australian magpie show rising anger. Note the extended feathers below and above the eye, which are normally sleek, accentuating the eyes. This kind of stare is a common form of reprimand to lower status individuals, such as male juveniles, without being followed by approach or attack if the juvenile changes its behaviour.

Fig. 9.8. Whole body expression of antagonism. A tawny frogmouth, sitting very still, raises its hackles and especially all wing and body. These wing and body extensions of the feathers on the abdomen and back double the size of the body. Note that the head feathers are not extended beyond the eyebrows and a ridge above the eyes. This male frogmouth spotted another large male in the distance and it is not clear whether this expression (without raising feathers around face and head) is a long-distance warning not to infringe territorial borders or whether this constituted a preamble for attack. The other tawny frogmouth flew away shortly after seeing this display.

Fig. 9.9. Head feather raising by kookaburras. (A) An angry kookaburra watches as a member of his own group snatches a piece of food from the ground ahead of this kookaburra that was about to fly down and collect it. Note the flare-up of feathers on either side of the top of the head – all other feathers remained sleek. The pilfering kookaburra consumed the morsel on the ground and chose not to join the group but stay on another tree, far enough away to avoid the wrath of the deprived kookaburra. (B) A juvenile kookaburra expressing fear when seeing a wedge-tailed eagle landing nearby, all feathers on the head are raised. (C) A painting by Jules Lewin – details of the raised feathers at the side and especially the back of the head in the early parts of an agonistic territorial display correctly show raised feathers as the black arrows indicate.

Fig. 9.10. Mobile crests. Cockatoos can have quite dramatic crests: (left) the palm cockatoo; (right) the pink cockatoo. The crest can be raised to any level, quarter, half, three-quarters and fully extended and each position may denote a different mood. Crests usually show states of affect and are erect on many occasions – just what they indicate depends on feather positions generally and body posture. In these images it is merely indicating a state of mild alertness.

Fig. 9.11. Fixed crest. Long-billed corellas have a permanent crest that can be raised further, extending the length of the head when viewed frontally.
Having shown a sample of a range of expressions involving the head or just the face, it is clear that many emotions can be made visible with very little energy. Having called these expressions ‘emotions’ is not an anthropomorphising of the birds discussed here. All of these expressions were followed by behaviour either of the sender or by another bird in such a way that it was possible to draw conclusions about their meaning. It was clear that these minute gestures had clear impact at least on one member of the group. In most cases, we expect those signals to be ‘honest’.
In the case of facial expressions and emotions, we cannot say whether they functioned as signals or that they were sent intentionally. In terms of communicative value, various pretend expressions may also be part of play behaviour. For instance, there is the mock flight intention (‘I am going to chase you’ or, at least ‘I could if I wanted to’) and I have even observed a case of a very well-acted display of anger that turned out to be a mock display (Fig. 9.12).

Fig. 9.12. Arching wings and back. Play anger in a juvenile magpie accompanied by loud mobbing calls. Note the arched back, lowered head and spread wings, also arched, and beak half-open. Seconds later, the bird sat up, relaxed and started preening.
Body postures as intentional communication
Body postures should generally be regarded as intentional tools of communication even if they express affect. In some cases, such body signals have become part of a territorial defence repertoire, as is the case in kookaburras using a distinct and sharp tail flick as a warning signal (Fig. 9.13). These could be displayed at greater distances and may be produced for instant effect. However, their ability to be visible at greater distances may also mean that these are used more exclusively for outsiders and even heterospecifics. It also implies that heterospecifics as well as conspecifics have somehow learned to read the signals correctly.
Intentional body signals as described here (as is the tail flick of the kookaburra) must not be confused with displays. Displays involving the entire body or only the neck and head may be adaptations that are innate and serve very specific purposes, such as for advertisement of sexual availability (males only) or even for defence. Displays may be very elaborate and lengthy and yet, as male song sung to attract a female, may say no more than ‘I am here, healthy and available’. To the best of my knowledge, Frith was the first who not only described the various body movements in native Australian pigeons but also provided wonderful drawings of them, especially the very detailed ones of the wonga pigeon (Frith 1977). These drawing have been reproduced in HANZAB (1996). In many cases in Australia, elaborate displays are part of a ritual for a long-term bonding that have developed into intricate joint ceremonies (well known in swans and grebes), involving coordinated dances, movements or even flight, as in little eagles. The daring and acrobatic flights by the little eagle were first described in great detail by Calaby (1951), involving dramatic rising into the air and plummeting at neck-breaking speed back towards the ground with folded wings and then opening the wings and repeating the same swing upwards. Such flight patterns have since been described also in wedge-tailed eagles (HANZAB 1993).

Fig. 9.13. Tail flick. The use of the tail for different display signals is quite widespread. When used as part of a display, it is usually a ritualised and adaptive form of communication. Tail fanning is often used in courtship displays but both examples here are outside that context. (Left) The tail-flick of a kookaburra, usually directed at kookaburras of another group to indicate territorial boundaries but here it concerned the response to a food item having been snatched from this bird. As a territorial warning, it is a clear and unambiguous signal. (Right) The tail-fanning by a grey-crowned babbler, also a cooperative group living bird. Another group member had landed very close to the first who indicated by the tail extension and forward crouching position that this patch was taken. The other bird hopped a few metres away and the tail feathers were lowered instantly. Alternatively, if the bird does not respond to the warning, the tail-flick will be repeated. Common koels also use tail flicking in conflicts between two males and white-winged choughs have multiple uses for the tail as a signal.
Other group rituals are used to consolidate bonds. Magpies fly together as a group and carol after a common enemy has been sent off their territory. Kookaburras ‘laugh’ together as an act of solidarity, usually in the face of a competing group. Apostlebirds come to sit close together in case of conflict and grey-crowned babblers have an unusual way of reconfirming their group bond by spontaneously coming together in so-called huddle displays in which they all fly to the ground, raise their tail-feather and jostle along as closely as possible, often accompanied by a chatter.
A highly unusual behaviour has been observed in the self-defence of white-winged choughs, seemingly with some variation judging by the descriptions provided by Rowley (1978) and 19 years later by Mackness and O’Brien (1997). In both cases, magpies were attacking a group of white-winged choughs. The two species tend not to tolerate each other because they both compete for the same food resources. In Rowley’s account, the white-winged choughs formed a circle, raising tail feathers and staring at the attackers (called a ‘plum pudding display’). In Mackness and O’Brien’s observations, the birds formed a circle but with their backsides out and the tail feathers raised right above their heads to form an umbrella. Eventually the magpies gave up attacking and the choughs dispersed. The group defence is ingenious and somewhat funny, thinking of all the cloacas displayed for the magpies’ benefit and the clever protection of head and neck by making the most vulnerable parts to attack disappear under a cloak of feathers.
These are standard repertoires and they too have a very important role to play as a regulatory mechanism for pair bonds and cooperative groups but have, by themselves, nothing to do with complex cognition. However, the argument presented here is that birds may use even established repertoire in individual encounters and do so only partially or with individual variations that are not for the purpose for which ritual displays were designed. In other words, a given repertoire may be extended and used for personal communication to express fear, anxiety, aggression or affiliative gestures.
This small selection of images depicting signals in real social situations demonstrates very clearly that socially living birds have a multitude of non-vocal signals that convincingly portray individual mood and emotions. These are effectively communicated because in all cases observed they led to a change of behaviour in a recipient and did consistently so. Such interactions tend to be just a few seconds long at a time but they are important regulatory mechanisms to keep the peace within a group, provided that every member heeds these signals while also remaining alert to other tasks. Group living thus adds an entire layer of interactions to everyday life, lending some support to the social brain hypothesis, as was explained before.
It is now also known that even autonomic responses can be modified by experience and instantly also by emotional reactivity, as has been shown in quails (Valance et al. 2007). Such mechanisms would need to be necessary to safeguard partners, offspring and cooperative groups alike and there is much that remains as yet unknown – for instance, why do groups not attack and kill each other at times of extreme stress and provocation? Emotional events modulate perception and attention. Selective attention (Schupp et al. 2003) may lead to interpretations that may require some kind of action and thus bring into play cognitive processes, such as decision making. The cognitive bias approach has now been convincingly applied to dogs (Mendl et al. 2010) but this does not explain the social brain and the willingness of individuals to actively or passively protect each other.
The case of the elevated cortisol level in a marmoset for days after the cage mate had fallen to his death, discussed above, is also relevant for cognition. If it were just emotional reactivity, the expectation would have been that the marmoset would have been distressed at the time of the event, partly because of the distress calls by the cage mate and partly because of the sudden fall. However, cortisol levels should have been back to normal within a few hours. That this was not so is revealing because the evidence of stress over several days suggests that the event was processed as something other than the fall. Whether the response contained empathy or was premised on an understanding of death or a sense of general loss or knowledge that the partner would not come back cannot be ascertained. All that can be said is that the response showed a good deal more than just affect.

Fig. 9.14. Diagram of a bird’s head. Literally every part of the feathers on the head can be used for communicating a particular affect. The cere refers to the fleshy part of the beak where the nostrils are and the lores are the area between beak and eye. Although this is sometimes important for identifying similar species or subspecies, they play a relatively limited role in individual expression, but the ear coverts do, as well as the forehead, the crown and the nape of the neck. The crest is situated on the crown. Eye rings may indicate age and even the pupil or iris may indicate a change of mood (an enlarged pupil can also be a sexual signal, as it is in tawny frogmouths).
The emotions shown here in some birds were all understood by the observing bird and therefore seem to have an important role at least in micro-management of daily life of partners or groups, if not for the longer term. To summarise, there is now some evidence that the avian face and head as a whole, are far more important in the communication of close distance communication in birds than has been acknowledged so far (Fig. 9.14). For lack of research evidence, this chapter has not been able to establish whether affective responses were voluntary or involuntary. One suspects that most responses in individual emotions are involuntary and honest. There are some important exceptions and, most importantly, it is the recipient needing to interpret the changes in mood and be aware of the other’s state of mind.
The most advanced research into emotions of birds and interactions at group level has been conducted on one species only: the common raven. Here the scientific envelope has been pushed further than was ever thought possible. Further to Table 9.1, investigations into the possibilities that birds may be capable of showing altruism and empathy are very recent. One of the most explicit papers on the topic of empathy is by Fraser and Bugnyar (2010b) showing that a distressed member of a group of ravens gets consoled by another member. Consolation for a vanquished member is of course also part of keeping a group functioning, but that such subtleties of emotions are possible to test has provided a new marker for the study of the entire range of emotional and cognitive abilities in birds.