11
So far, many of the special abilities of native birds described in previous chapters might be dismissed as adaptations or based on simple associative learning and not as evidence of cognitive complexity. Although problem solving is without doubt an advanced ability, skills such as tool using and even imitations are actions that could be a long way off from complex thought. At least in the 1990s, the question: ‘when does behaviour reading become mind reading?’ (Whiten 1996) could not be answered satisfactorily. And single skill evidence raised the question: ‘if an animal shows an aptitude in one specific area displaying abilities that were once considered unique to humans, does that mean that such an animal or species is intelligent?’ There was a tendency to answer with ‘yes’ when great apes were concerned as the nearest human ancestors and ‘no’ in case of all other vertebrates, a position that has since been shown to be an untenable a priori position (Kaplan and Rogers 2004c, Shettleworth 2009a).
For instance, the North American Clark’s nutcracker, a member of the corvid family, can hide tens of thousands of nuts and then recover most of them, even after snow has fallen and any local landmarks have disappeared. Such spatial memory using geometric cues is extraordinary (Shettleworth 1990) and probably without parallel, but is it ‘intelligent’ or could this be explained as a necessary adaptation encoded in the genome of the species itself? If so, then the idea of intelligence is fanciful because this behaviour demonstrates that the bird has evolved to meet specific challenges of its environment, that it can do things spontaneously and, importantly, without thinking about the problem at hand (Martin and Bateson 1993). And even the clever ability to imitate may just be based on the well-worn, known and much simpler mechanisms of associative learning (Catmur et al. 2009).
There is now also plenty of evidence that vertebrates are born with certain predispositions expressed in the visual and auditory system that, however rudimentary, may help an organism to respond to threats and affiliations in the environment. For instance, experimental evidence suggests that biologically relevant stimuli, whether acoustic or visual, such as large frontally placed eyes, the shape and movement of a snake, or the deep growl of a large cat trigger responses of fear in completely naïve vertebrates, including birds (Beránková et al. 2014). Animate creatures are another important class of biologically relevant stimuli. Who recognises whom, and how quickly, can be a matter of life and death and a recent study using chickens has identified detection of animacy as an important step for the development of the social brain (Rosa Salva et al. 2015). Hence, the studies in auditory and visual perceptions and the associated brain mechanisms may be rather central to understanding an animal’s responses (Wylie et al. 2009; Wilson and Lindstrom 2011).
The brain mechanisms that are needed to enable innate responses are now also better understood and evidence has shown that these mechanisms have been present and well conserved in vertebrates over a long evolutionary time (Sewards and Sewards 2002). It is a matter of identifying how interaction with the environment may enhance these predispositions and, finally, at what point a response by an individual is not merely the result of adaptations of brain mechanisms that require no thinking, but clearly based on additional cognitive processes.
Advances in cognitive ability might well have happened in minute incremental changes in function, along with expansion of the brain. This may not only relate to long evolutionary periods, but such changes can also be sudden and be evident even within an individual’s lifetime. For instance, in cooperatively breeding white-browed sparrow weavers, social group life involves dominance hierarchies with sufficient openness so that male subordinate helpers can occasionally advance to dominance and, in such cases, the male in question also changes his song and with it the volume of the song control area (HVC) in the brain and their gene-expression (Voigt et al. 2007). Such plasticity may be impressive, but that too is not necessarily an example of intelligent behaviour.
To demonstrate a general intelligence, it has been argued, animals would have to show manifestations of memory (making and maintaining memories – spatial, acoustic, seasonal, social), abilities for concept formation and categorisation, remembering things that have gone out of sight, as well as be ‘time travellers’: that is, not only be able to remember the past (perhaps even grieve for a conspecific) but also plan for the future in the short and even the long term. Further, an intelligent individual is meant to be able to judge what another feels or thinks, which is called ‘inferring the state of another’. This may include behaviour such as eye gaze following (‘I want to see what you see’), pointing or responding to pointing (‘I want to show you something’): expressions of intentionality and coordination should be apparent.
In this list of abilities and specific modules that make up ‘intelligence’ is the so-called ‘theory of mind’ (Shettleworth 2009b, 2010). An organism can be said to possess a theory of mind when it is capable of attributing mental states to others – when it understands that others can see, feel and know – and this is inextricably linked with a capacity for consciousness, insight, a concept of self, and such emotions as compassion and even pride and shame (De Waal 2011). Of course, all these abilities were thought to be well beyond any animal, let alone birds, and even now many of the abilities when tested under rigorous conditions are met with great scepticism (Penn and Povinelli 2007; Penn et al. 2008).
The idea that memory of past events exists in animals is no longer under dispute, but the question is for how long such a memory may last. In the example of the currawong that I had hand-raised (see Chapter 3), there was irrefutable evidence that the bird recognised me and also remembered the dish of grapes in a hidden spot and was able to access this memory spontaneously. Unfortunately, longitudinal research is rare in bird studies and hence the absence or presence of very long-term memory in birds is so far largely a matter of conjecture. However, I have had evidence of long-term memory in galahs and sulphur-crested cockatoos for the worst reasons. A number of pet birds were passed onto me that had obviously been abused (broken bones in legs and wings, etc.). In almost all cases, it was possible to reassemble either some criteria about the type of abuse or the characteristics of the abuser. In one case, through a series of trials with different objects, clothing and head gear, the identity of a person feared could be established and by asking the most recent previous owner it could be confirmed that the type of person the bird feared to a point of panic (and loud screeching in fear and hyperventilating) dated back 20 years. He had simply never forgotten his tormentor. Perhaps, birds can retain long-term memories of specific events for a lifetime. In this case it was fear. In the famous case of Koko, the gorilla, it was sadness. In sign language, as already mentioned, she expressed grieving for her lost friend, a kitten (McGraw 1985).
It is also clear that, very early in evolution, the brain developed specialisations and one way in which it did so was by using each hemisphere for different functions. This is called hemispheric specialisation, meaning that the left side of the brain attends to different things (or to different qualities of the same thing) than does the right side and it controls different functions and behaviour. Once this was thought to be a unique characteristic of the human brain but we now know that it applies to other animals. For example, recently honeybees have been tested for their ability to memorise certain odours and researchers found that long- and short-term memory was encoded on different sides: the right antenna is used to recall short-term memory while the left antenna is used for recall of long-term memory (Rogers and Vallortigara 2008). Remarkably, it has now been found that the right antenna in bees is also used to control appropriate social behaviour (Rogers et al. 2013). The storage of memory on different sides of the brain (here the antennae) is referred to as lateralisation. Its importance has only recently been fully appreciated (Corballis 2014).
Brain lateralisation and survival
It is no longer under dispute that brain lateralisation has a very long evolutionary history. It has been tested and found in invertebrates, as mentioned above, and been shown in many birds and mammals. Countless papers now exist on the various aspects of lateralisation of the brain (Rogers et al. 2013), but there are still relatively few that explore its importance for survival in animals. In 2000, Rogers began to ask what advantages a lateralised brain might have for survival in animals in general (Rogers 2000). A set of experiments followed that highlighted crucial differences in learning and in dealing with multiple stimuli (such as searching for food and monitoring for a predator overhead). The domestic chicken was an ideal model for such experiments. It was already known that dark-incubated chicks were non-lateralised for processing visual input. That is, the mere exposure of the eggs to light for only a brief period during the final days of incubation ensured that the chicks developed fully lateralised brains, but those eggs that were artificially incubated in continuous darkness produced chicks without brain lateralisation. Hence, the hypothesis that hemispheric specialisation of the brain is an advantage to survival could be tested under very tight experimental conditions. One experiment compared the performance of light-exposed and dark-incubated chicks on a pebble floor task (in which the pebbles of similar size and colour as grains were stuck to a floor) with grains scattered across the surface. The chicks in both groups (light and dark incubated) had to learn to discriminate between the pebbles and the very similar looking grains. While they were pecking, the silhouette of a bird of prey in flight was moved overhead.
The dark-incubated (non-lateralised) chicks detected the raptor overhead significantly later than the light-exposed (lateralised) chicks and they became increasingly distracted and confused by the conflicting demands of the test and made many errors in pecking, securing fewer grains than the other group. Next day, the non-lateralised chicks showed no memory of the performance of the task (i.e. their performance had not improved). By contrast, the lateralised chicks detected the overhead raptor faster than the non-lateralised chicks and showed clear improvement of performance in the pebble floor task. They had learned and had formed a memory of the task.
This was the first demonstration of the importance of hemispheric specialisation for survival (Rogers LJ et al. 2004) and showed how performing more than one task at a time is related to lateralisation. Furthermore, Regolin and colleagues found that storage of working and reference memory depends on the tasks or objects to be remembered: ‘what’ and ‘where’ information may be retained in both hemispheres while the right hemisphere is dominant when conflicting information exists between object or position-specific information (Regolin et al. 2005).
The function of lateralisation in birds, other than in typical laboratory species such as domestic chickens and pigeons, has remained largely untested. There have only been a few papers, with one on the black-winged stilt (Ventolini et al. 2005) showing that copulation in stilts was predominantly on their left side and they used the right eye for foraging when trying to locate fish under water. Just as domestic chickens use different eyes for these separate tasks of foraging versus copulating, the results of the stilt study showed that specific viewing events are processed not just by a different part of the brain but also by a different side of the brain (Ventolini et al. 2005; Marzluff et al. 2012).
In 2007, our laboratory also investigated hemispheric specialisation in a predator-related task that we set up in the field, making observations on free-ranging non-provisioned groups of wild Australian magpies (Koboroff et al. 2008). This was the first native Australian bird ever tested in the field and only the second worldwide. We placed a taxidermic model of a monitor lizard – a known bird predator of eggs, nestlings and juveniles – into a central position of the group’s territory and then scored the magpies’ eye preferences, while they approached the model and while they engaged in anti-predator responses. Significant eye preferences were found. These eye preferences, incidentally, can tell us rather precisely where in the brain the information is processed. The left eye information is processed largely in the right hemisphere and the right eye input in the left hemisphere.
Eye preference scores showed that anything to do with approaching the model was viewed with the right eye (i.e. the left hemisphere), while the left eye (right hemisphere) was used for 85% of the time prior to moving away (escaping) from the model (Koboroff et al. 2008). Fear and escape are controlled by the right hemisphere, as they are in other vertebrate species (Lippolis et al. 2002, 2005). Mobbing of the predator, as well as inspecting it, was done with a left eye preference (right hemisphere). Incidentally, chickens process human faces and eye gaze also in the right hemisphere (Rosa Salva et al. 2007). The evolutionary implications are two-fold. The relationship between these hemispheric specialisations and predator–prey interactions suggest that a suite of anti-predator strategies may have been organised within the right hemisphere; and, from the results of the chicken, it can be inferred that the stronger such lateralisation is, the swifter and more focused the ability to respond (Rogers et al. 2004). The persistence of anti-predator behaviour further suggests that such specialisation developed early in evolution (Blumstein 2006).
The question arises whether lateralisation is also of some importance in anything that might be considered cognitively complex and this too has recently been tested. We have already discussed the means-ends test in the chapter on tool using (Chapter 4). Food out of reach, but on a string, could be hauled in if a tool or foot was used.
In a set of experiments using eight Australian parrot species, the means-end tests were also employed, but with a different set of questions in mind. The researchers (Magat and Brown 2009) wanted to know whether lateralisation (and its strength) showed up as an important variable correlated with performance in the cognitive task of the means-ends test. They used cockatiels and budgerigars – two Australian species known not to use their feet for feeding and not to have a foot preference on other tasks (i.e. they have no ‘footedness’ but may otherwise be weakly or strongly lateralised) – and six other species that do use their feet. Four of them were cockatoos: (galah, gang-gang cockatoo, red-tailed black-cockatoo and sulphur-crested cockatoo), and two were parrots (Australian king parrot and superb parrot). First they determined foot preferences in manipulating food objects or in the manner of standing on a perch while feeding (Fig. 11.1). They also scored which eye was used to fixate the food and put those two measures together to get an index of lateralisation. Note that foot preference correlates with eye preference (Brown and Magat 2011).
Their first experiment replicated the pebble floor task used in Rogers’ experiments with domestic chicks to discriminate between the pebbles and grains. In this experiment, red-tailed and sulphur-crested cockatoos outperformed every other parrot species, followed by galahs, gang gangs and cockatiels and the least well performing group (budgerigars, the king and superb parrots) among which there were no significant differences. Strength of lateralisation (eye preference) showed up as a significant variable: performance was better in species with stronger lateralisation.

Fig. 11.1. Left foot food manipulation. Cockatoos can use their feet to manipulate food: (left) sulphur-crested cockatoo; (right) pink cockatoo. It was discovered that they use mostly the left foot (Rogers 1980). Such use of the same foot for feeding shows lateralisation, or footedness, in cockatoos and this correlated with lateralisation of eye preference (Brown and Magat 2011). Different techniques are shown in this figure: the sulphur-crested cockatoo is using the foot to anchor down a food item, while the pink cockatoo is lifting the food item to the beak. Note that food is held between the first and second digit but not grasped from the inside of the foot but from the back. The grip is surprisingly powerful, enabling the bird to firmly hold very small items such as a nut and very large items such as an entire banana or a seed cone of a banksia, irrespective of the size of the cockatoo (i.e. it is a well-developed adaptation).
In the second experiment, food was suspended on a string (means-end test). Neither the budgerigar nor the cockatiel could solve the task on 10 trials. However, all six remaining species could, although there were differences between them, depending on the strength of lateralisation. The important finding was that the most strongly lateralised individuals (according to a laterality score) were also the most successful ones in solving the means-end test. Again, as in the chicken experiments involving a conflicting set of stimuli (food below and predator overhead), the strength of lateralisation determined the degree of success of the outcome.
There is another advantage of lateralisation that has been identified and it is of more than marginal importance. Rogers and Workman (1989) found that strongly lateralised chicks formed more stable hierarchies in groups than those that were weakly or not at all lateralised. Hence, for group living, lateralisation seems to have a direct influence on social life or vice versa: social life may only develop because the brain processes facilitate such a development and continued interactions lead to population biases (Ghirlanda and Vallortigara 2004). Bearing in mind our interest in Australian cooperative species, it would be interesting to know whether birds, such as apostlebirds or white-winged choughs, known as intensely communal and cooperative, have strongly lateralised brains, or, in the large family of thornbills, in which some breed cooperatively and others do not, whether there is a difference in the strength of lateralisation.
Systems of knowledge
Some areas of cognitive science now tend to avoid speaking about the potential for intelligent decisions and instead examine animal cognition under rubrics summarised as systems of knowledge. What is it that an animal needs to understand (or know) about the world around it so that it can function and how can it improve its life merely by adding knowledge to an existing system? Cognitive scientists distinguish between several core knowledge systems, such as those related to physics, mathematics (Rugani et al. 2009), geometry and psychology. The debate is very well summarised in a recent review (Vallortigara et al. 2010). Concepts such as cause and effect in physical tasks and visual cognition, including object permanence (explained below), may be part of the core requirements of knowledge.
The reference to a core mathematical knowledge may sound puzzling, but some elements of basic numeracy must be core knowledge. Imagine two separate groups of geese (or chickens or ducks) moving out of sight, one to the left and the other to the right. One group consists of five individuals, another of three, all moving in file behind a barrier. The last gosling has to (a) remember that its family is just behind the barrier and (b) it has to make a decision as to which is its own group. One of the distinguishing features is a numerical one. Invariably, the gosling will choose the correct number and hence its own group. Indeed, it is so basic that this ability has been found in newly hatched chicks (Rugani et al. 2009, 2010b) and even in insects (Dacke and Srinivasan 2008). In birds that suffer brood parasitism by cuckoos, the ability of counting the number of eggs that ought to be in the nest, would also reduce brood parasitism and does so in a number of species (Lyon 2003). Numeracy has also been tested in jungle crows (Bogale et al. 2011b), in pigeons (Hirai and Jitsumori 2009) and in African grey parrots (Pepperberg 2006a; Ain et al. 2009). The same abilities have been tested in primates and dolphins (Kilian et al. 2003) but, to my knowledge, not in Australian birds. Interestingly, birds (chicks and Clark’s nutcrackers) have also been tested on ordinal positioning and it has been found that they can not only order objects from lower to higher but also do so from left to right (Rugani et al. 2010a). This leftward bias assumes that the lower number is on the left side.
A more advanced type of physical knowledge is that stones can displace water and make the level of the water rise. Some of the most impressive examples have been found in common ravens that used stones to raise the water level in a narrow glass to be able to retrieve a worm floating upwards as the water level rose (Bird and Emery 2009). This ability has also been documented in orang-utans. Orang-utans used water to make a nut float to the surface of a narrow glass by fetching water in their mouths and spurting it into the tube that contained an otherwise unreachable nut at the bottom of the tube (Mendes et al. 2007). Finding such solutions to a problem that demands knowledge of physical causation was previously thought to be impossible and improbable in animals generally, let alone in birds.
The string pulling and retrieving food problem, already mentioned, is a physical task that, other than understanding means and ends, relies on core knowledge of gravity if the food is suspended vertically from a branch. Keas have been tested on similar sets of tasks and, without hesitation, solved the problem on each occasion. More remarkable, perhaps, was the case in retrieving food from a box with locks and complicated mechanisms, in which the New Zealand keas did just about as well as chimpanzees (Auersperg et al. 2011; Miyata et al. 2011).
However, in a task that demanded manipulating a food item past a trap (the trap-tube task; Fig. 11.2), parrots apparently failed, including keas and a cockatoo, while apes and corvids succeeded (Seed et al. 2006, 2009; Liedtke et al. 2010). The trap-tube task may well have become a benchmark test for investigating knowledge of physical causality in vertebrates or, rather, for intelligence that requires grasping physical causality (gravity), but perhaps this test has a flaw in that it may not be suitable for birds with large curved beaks. Australian parrots are not nest builders, except for the ground parrot (Lindsey 1998) and their curved beaks are not designed for stick or tool manipulation, particularly if this includes negotiating narrow spaces. Many of them, as was already shown, use their feet for manipulation and ought to have been given alternative ways (via feet) to retrieve the food. Hence, one cannot tell from the results of such a test whether the failed outcome was the result of physical limitation rather than a lack of understanding of the problem. In the very least, the results should caution us to rethink what we term tests of cognition (or intelligence).
A test that does not require manipulation with the beak was used for keas as an enrichment item at Auckland Zoo in New Zealand and it shows that keas can understand the concept of gravity. A box with partitions in the form of simple interspersed partial wooden barriers and with a clear plastic cover was placed upright. In front of the box, a platform was appended to a rolling post underneath, hence enabling seesawing from left to right with the entire box when tipping the balance from one end to the other. At the top of the maze the keepers placed a nut or a grape. Using a seesaw action of changing of body weight (e.g. from one foot to another) could get a grape or nut to roll downwards from one level to the next and make it come out at the bottom of the contraption (Fig. 11.3). Keas were able to do this successfully and thus showed that they had understood the causality both of gravity and the maze and, on evidence, it seems that they understand how their own movement influences the direction of movement of the food item. How the bird got to perform this task and so well, is not known. It would be a test that could be used easily on Australian psittacine species for comparative purposes.
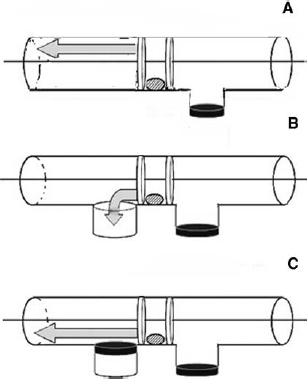
Fig. 11.2. Apparatus for the trap-tube task. Arrangement A shows a classic model used for working with great apes (Mulcahy and Call 2006); arrangements B and C were used in the experiments using birds (Teschke and Tebbich 2011; Teschke et al. 2011). The apparatus for the trap-tube task is very simple. A piece of tubing contains a favourite piece of food. The food can be obtained in a number of ways via an implement provided, be it a stick or a rake or a plunger. The decision the animal has to make is whether to push it away or rake it in. That decision needs to be tempered by an understanding of what the hole with a trap below the tube means for a successful retrieval. The animal has to anticipate that the food will drop and disappear if pulled or pushed across the hole. The diagram shows just three variations of the tube task and the arrow indicates in which direction the plunger, and so the food item (the small round shape in the middle of the tube), ought to be pushed or pulled for a successful retrieval.
Another separate problem has been investigated traditionally within the Piagetian conceptualisation of ‘object permanence’ (Vallortigara 2004), as the first step in intellectual development and as a prerequisite for any meaningful interaction with the environment. Piaget argued that it was an important conceptual step to grasp basic rules of physics, mathematics (Rugani et al. 2009), geometry and psychology and when individuals are able do this they can also comprehend their own identity as a separate entity among all others, and can then situate themselves in time and space (Piaget 1953, 1954). Although conceived as a framework for the development of human intelligence, his six-step developmental outline was readily transferrable to animals and, since the 1980s, had spawned a multitude of experiments.

Fig. 11.3. Understanding gravity: keas with tilting maze. Once the kea has looked at the layout, it steps onto the platform, taking a centre position above the rolling post and literally rocking from one leg to the other until the grape has rolled out of the opening on the lower right hand side. This kea is just stepping onto the platform. (Image modified from Auckland Zoo Kea Behavioural Enrichment Manual 2004 – available online.)
The problem of thinking of objects that are out of sight was previously described in the hide-and-seek game of magpies (Chapter 6) and was once thought to be unique to humans. Quite a number of experiments with chicks have shown that even young chicks can do this and can hold and use memories of things hidden (Regolin and Vallortigara 1995). Eurasian jays (Zucca et al. 2007), European black-billed magpies (Pollok et al. 2000) and carrion crows (Hoffmann et al. 2011) also showed object permanence. African grey parrots, as well as budgerigars and Australian cockatiels, have also been tested on various tasks relating to object permanence. On a number of occasions, they solved the tasks that were assumed to be more difficult before they attended to the easier ones (Pepperberg and Funk 1990). However, the number of bird species tested other than parrots, pigeons, chicks and corvids remains very small and does not compare to the wealth of papers on the subject in primates.
An anecdote of a magpie group would suggest a deeper insight into hidden objects well beyond basic physical core knowledge and it is worth recounting here in full. When I had set up a research site to observe a permanent residential group of magpies on the Northern Tableland of New South Wales (near Armidale) and the group was familiar and comfortable with my presence, I had decided to build a hide so that it would not be clear whether or not I was present. When the hide was nearly complete and was tested for comfort and visibility, one magpie suddenly appeared at my feet, slowly and deliberately walking around the barrier to the entry door, being very circumspect and constantly looking around and up at the inside of the hide. It then flew up to investigate the viewing slit in the hiding box and sat down on the lower rim of it, followed by flying down and up again at the slit. Suddenly (at least for me), the mood changed and the bird issued a series of alarm calls and its posture changed entirely to an angry attack posture (wings out, head lowered and eyebrows and feathers raised). Other magpies came and the angry magpie started to take swipes at the hide. It was made only of mesh and plastic, stapled on to a wooden frame. The colours of the mesh and corrugated sheets for the sides were green and the roof a non-descript grey colour typical for old corrugated iron sheets. Within minutes the group of three adult magpies had shredded the hide completely and left me, as a stunned observer, next to the demolished hide while they flew off.
What can one possibly make of this scene? There was nothing in the colours or structure that I thought could possibly have alarmed the birds. It happened when the task was nearly finished and I had entered the enclosed hide for the first time and had thus completely disappeared from view. The magpie that investigated my disappearance must have followed my movements closely because it was right next to me the minute I stepped outside the hide again. The magpies easily ripped the mesh from the staples and the hide was left in tatters but, interestingly, they did not attack me. I did not build a hide again in magpie territory. The group continued to keep a close eye on my movements but out in the open. Could it be that their games of hide-and-seek presupposed an understanding of ‘hiding’ and an understanding of risk in anything hidden? Cognitively, it seemed to be extraordinary and took my breath away. Such questions cannot be answered without tight experimental controls. The group later even allowed me to stand on a guy-roped ladder within 10 m of their nest without attacking or attempting to swoop.
It is worth remembering at this point that the systems of knowledge described above as the proposed units for analysis in the evolution of cognition are increasingly being shown to exist in insects, if in simplified form. Bees, for instance, also have spatial memory and relational concepts; indeed, they have a basic numerical cognition (Collett et al. 2006; Dacke and Srinivasan 2008). Far from being unique to humans or extended only to the primate line and some clever birds, such as Alex, the African grey parrot (Pepperberg 1994), it appears that certain categories of physical knowledge were so necessary for finding food and/or kin that evidence of such abilities now found in bees and other insects (Pahl et al. 2013) needs to be discussed in these broader terms and traced through evolutionary history.
Eye gaze following
Eye gaze following is usually classified as psychological core knowledge. Group living may demand an expansion of unambiguous signals, intentionality of purpose and often also of status clarity. Wondering about what another individual is thinking or seeing may be shown in following the other’s direction of eye gaze. The simple questions: ‘what are you looking at?’ or ‘what do you see?’ or even ‘why are you looking there?’, express an aspect of higher cognition because it presupposes that the observer is aware of the state of another and interested in someone else’s frame of mind.
Apart from work on viewing in chickens by Marian Dawkins (1995, 2002) and by an Italian research team at Trento and Padua (Rosa-Salva et al. 2007), African grey parrots (Giret et al. 2009), bobwhite quails (Jaime et al. 2009), bee-eaters (Watve et al. 2002), Northern bald ibises, jackdaws (Von Bayern and Emery 2009) and starlings (Carter et al. 2008) have been shown to engage in eye-gaze following, as had been found in primates. Common ravens also cache food items and they might use the ability to recognise direction of gaze to decide when they are being observed as they cache it (Bugnyar and Kotrschal 2002; Schloegl et al. 2007).
Hand-raised common ravens were tested to see whether they could follow the direction of a human’s eye gaze and it was found that not only did the ravens look up when a human looked up but also, when the human was looking at something hidden from the raven’s immediate view by a barrier, the raven would come over to the barrier and peer around it (Bugnyar et al. 2004). If the raven was responding only to simple cues, we would expect the bird to do no more than stay where it is, look at the barrier, find it uninteresting and go on with whatever it was doing before. Instead, the raven behaved as if it was aware that something interesting was located behind the barrier.
Of all the species tested, only bee-eaters occur in Australia (Watve et al. 2002). We have no studies of this kind in Australia to show such behaviour under controlled conditions; however, it would be difficult to believe that mutual watching and eye gaze following were not also part of the way in which cooperative groups have evolved. Eye gaze can thus clearly be used as a directional cue. It seems it is one that species of many different classes can grasp, while a directional cue given as a gesture is limited to species that can use a limb to do so.
Pointing
As part of a gestural repertoire in humans, pointing has often been identified as a key behaviour for understanding development of language and a theory of mind (Camaioni et al. 2004). Pointing has been studied in great detail in apes and children, but not in other species. Quadrupedal animals, such as dogs and horses, or animals such as dolphins or birds, do not have limbs that can be used for pointing and hence pointing was not considered possible nor was it thought that they would understand the meaning of pointing. This has proved not to be quite true. Some studies found that domestic dogs and guide-dogs (Ittyerah and Gaunet 2009; Lakatos et al. 2009), wolves (Virányi et al. 2008), horses (Maros et al. 2008) and dolphins (Xitco et al. 2004) can be trained to understand the meaning of a pointing gesture by humans whatever the context. Moreover, it was found that head-turning or whole body turning and even eye gaze and eye-gaze following (Anderson et al. 2007; Kamphius et al. 2009; Kaplan and Rogers 2002) can replace pointing with the same cognitive processes coming into play.
The existence of vocal referential signalling in birds had already been explored and experimentally verified (Evans 1997; Kaplan and Rogers 2013), but had not previously been extended to gestures. Nevertheless, it is not far-fetched to hypothesise that referential signalling could be extended to referential gestures. Indeed, it was one of those special moments when I discovered pointing in Australian magpies. Casual observation of a behaviour that looked like pointing behaviour was followed by a set of controlled experiments in the field. I had noticed that a magpie issued its referential ‘eagle alarm call’ but was also posturing in a specific direction and when I followed that direction, I saw an eagle on the ground. It flew away shortly after I had spotted the eagle, so the impression was fleeting. However, I then set up experiments in the field, using a taxidermic model of the same species of eagle, a wedge-tailed eagle, providing a number of specific testing conditions involving different positions of the eagle, in the open to semi-hidden under low scrub (Kaplan 2011). In the hidden condition, the results showed that the magpies not only engaged in their referential alarm calling but also used a distinct pointing gesture to warn other magpies in their group of impending danger. As the others arrived, they first followed the pointing magpie’s gaze and then they too adopted a very distinct body posture and aimed their entire body in the direction of the eagle until the next magpie flying in to investigate had also seen it, then also vocalised and pointed (Fig. 11.4).
Pointing can be for entirely selfish purposes and this is usually the level at which infants first start pointing (‘I want this toy’, ‘I want this food’ and ‘I want you to get these items for me’). It is a much more complex action to gesture about danger not just to oneself but also to others and to expend considerable energy to ensure that others understand this. The pointing magpies did not point to get something for themselves (such as in begging for food gestures), but to let other birds know of the presence of the eagle. I concluded the paper by saying that the discovery of beak pointing in magpies stands in a very long tradition of debate on pointing, cognition, speech production and hemispheric specialisation, but almost entirely on its own in research on birds (Kaplan 2011). It was the first time that pointing behaviour had been reported in a bird.

Fig. 11.4. (A) Pointing in magpies. The wedge-tailed eagle is Australia’s largest eagle: a female can weigh about 6.5 kg. Their usual quarry are rabbits and joeys but they will occasionally also kill birds, including even magpies. (B) The magpie, perched high on a branch spots an eagle standing on the ground and partially hidden by shrubbery, then issues a referential eagle alarm call and on arrival of another magpie adopts a posture in which head and body form a straight line in the direction of the eagle – the observing magpie has to follow the direction of pointing. (C) Once other magpies have also spotted the eagle, they then also adopt this posture for the benefit of the next arriving magpie. (For further details see Kaplan 2011.)
There are now two papers on pointing birds. In the following year, Pika and Bugnyar (2012) published a paper showing, also in field studies, that common ravens engage in gestures of pointing toward objects of mutual interest. Hence, pointing is a sophisticated form of communication that has precursors not just in the primate line but also in the evolutionarily distant class of birds.
The question asked by Whiten in the 1990s: ‘when does behaviour reading become mind reading?’ (Whiten 1996) can be answered nearly 20 years later. Pointing and eye gazing involve not so much reading the behaviour of another but interpreting what the other might think it sees that is important.
Body movements, particularly when only showing pretended actions, can also serve as important markers that require an observer to interpret an intention. This is different from imitation of an action or eye gaze following because the action shown is incomplete and the onlooker has to make the important cognitive leap that it is not behaviour the demonstrator is doing but an action that the demonstrator wants the observer to undertake. For instance, deliberate gestures can be used in teaching contexts.
Kookaburra females do this by showing, not feeding, youngsters enticing food morsels from a close distance. At the same time, the female flaps her wings. This is not to show her own flight intention but to persuade the youngster to make an attempt at short distance flight (Fig. 11.5). Such deliberate acts for the purpose of effecting a change of behaviour in another clearly belong into the realm of complex cognition.

Fig. 11.5. Kookaburra gesturing to offspring. This frame from a video recording shows a rarely seen behaviour of gesturing as an intentional signal. A kookaburra parent is trying to encourage a newly fledged offspring to fly by gesturing flight movements and showing a large morsel of food just out of reach of the youngster to entice the fledgling to move. (Taken from author’s recordings.)
‘Time travel’
Time travel is perhaps an awkward term to describe that birds may need to be aware of the ‘what, when and where’ of any important facets of their lives (such as predator or food). If a bird found a good food source, for instance, it may need to remember where it was cached or found and plan to return to it in future. Dimensions of time from past to future are an important addition to the list of research interests in cognitive abilities in birds, generally referred to as episodic-like memory. This is not quite the same as ‘time travel’ or ‘mental time travel’, which, in human research, refers to the ability to imagine a future or a past. In birds, it usually concentrates on the acquisition and storing of food. It took researchers a long time and plenty of research evidence before it was finally conceded that birds can remember past events and perhaps even plan for the future (Emery 2006). Goto and Watanabe (2012) found that large-billed crows have retrospective but not prospective metamemory while the research by Clayton and Dickinson (1998) had shown that birds can indeed plan for the future. It had always been thought that animals can only live in the present and respond to current needs and events. However, nectar-feeding animals must know that their food source gets depleted during the day and also when it is replenished. We have already seen (Chapter 3) that feeding on flowers is not just the province of nectarivores. Apart from honeyeaters and lorikeets, quite a number of Australian birds, not considered nectarivores, also partake of nectar. There is evidence that animals relying on nectar as their main or sole source of nourishment, such as bumblebees (Boisvert and Sherry 2006) and hummingbirds (Henderson et al. 2006), can learn the intervals at which this food source replenishes. Their memory must include information about what flowers they visited, where they were and when they last fed from them.
Another rather large group of birds store or cache food. Some storers, such as woodpeckers, keep a larder that is clearly visible, but Clarke’s nutcrackers and many other corvid species hide their food. In the latter group, caching has some additional variation that they may cache both very perishable and less perishable foods. Hence, the time factor can become very important and the more perishable food should be retrieved before the non-perishable food. Corvids, including jays, have been studied extensively in this regard (Clayton and Dickinson 1999; Grodzinski and Clayton 2011), but so have a few other species including the black-capped chickadee (Feeney et al. 2009). Retrieval of cached items may mean that the bird uses a cognitive spatial map: that is, uses geometry rather than simply the details or landmarks surrounding the spot where each seed was cached (Shettleworth 1990; Jones et al. 2002; Emery and Clayton 2004). Retrieval of the perishable food first requires that a time element is included.
Given the extensive number of research papers on complex bird behaviour, such as caching, in North America and in Europe going back well over three decades (Smith and Reichman 1984), it is disappointing that Australian species have so far not been fully integrated into international debate on caching and what it implies, because there is plenty of evidence of caching in Australian species as well. Interestingly, in Australian birds this behaviour is not confined to corvids but has been observed also in the Artamidae, to which butcherbirds, currawongs and Australian magpies, as well as woodswallows, belong. Except for woodswallows, all other Artamidae appear to use caching, but this has usually not been a matter of systematic investigation but of anecdotal reporting, as in the grey butcherbird for instance (Walters 1980). Caching behaviour in magpies and currawongs (Reynolds 1969) has already been mentioned (see Chapter 3). Above-ground (tree-caches for instance) and terrestrial caches have to be distinguished too. Butcherbirds and shrikes seem to cache largely in locations well above the ground. Walters described a single grey butcherbird first securing a piece of meat on a household television antenna placing the piece in a join of two segments of the antenna, then returning for another piece and securing it in a power line insulator fixed to a neighbouring house and a third piece in the fork of a tree. Each cache was at least 20 m apart from the next (Walters 1980). In 1978, the journal Emu published a short communication reporting the caching of food by Torresian crows (Chapman 1978), also caching in an elevated position. The report describes a group of three Torresian crows, including one juvenile, taking turns in caching the food in repeated flight missions up steep cliffs at Mt Olga in the outback region near Alice Springs. In a period of a mere 30 minutes the crows had either consumed or mostly cached in rock crevices over 1 kg of bread (Chapman 1978). That same year, two further publications reported caching in ravens: one observing forest ravens (Leonard 1978) and another on caching behaviour in little ravens (Lewis 1978). A year later, a further brief communication reported Torresian crows burying and hiding food (Walters 1979) and these were all ground caches. However, Secomb (2005a) described in detail that forest ravens forage in trees and cache their food.
Of all Australian corvids species, three are widespread: the Australian raven is distributed throughout the eastern part of Australia and in southern Western Australia; the Torresian crow in the north and centre; and the little crow can be found throughout much of Australia except for the eastern seaboard. The forest raven is largely confined to Tasmania (with a northern subspecies found in north-eastern New South Wales; Debus and Rose 2006), while the little raven is exclusive to the southern parts of South Australia, to all of Victoria and parts of New South Wales; that is, every state and territory in Australia has at least one corvid species, but some areas have as many as three (Barrett et al. 2003; HANZAB 2006b).
The number of systematic studies on Australian corvids about their biology and ecology have slowly filled the gaps, starting with Rowley’s 7-year CSIRO-funded study of Australian corvids (Rowley 1973), followed much more recently by studies on forest ravens (Secomb 2005 a,b; Debus and Rose 2006; Lawrence 2009; Talmage 2011), little ravens (Talmage 2011; Cardilini et al. 2012) and Australian ravens (Lee 2011), but any systematic work on cognition in the Australian corvids, whether in the field or in laboratory experiments, is still largely absent.
In northern hemisphere species, be this in Europe or in North America, caching has often been found to be associated with questions about a bird knowing the state of mind of another. They observed that many corvid species not only hide their food but other birds in the same group watch them hide it and then try to steal it. So the bird trying to hide food has to be aware that, if watched by a conspecific, it could lose its stash. If it is clever, it will re-hide the item once the observer has gone. Indeed the western scrub-jay does exactly that. It may even get more complicated in that the original bird has to remember when and where it had seen the other watch it hide the food (Dally et al. 2006a,b) or to conceal auditory information just so that a competitor cannot hear it during the re-caching (Stulp et al. 2009). The latter was also found in Eurasian jays (Shaw and Clayton 2013). If a human actor were to show this behaviour, we would say that he or she knows what is on the observer’s mind. Primatologists refer to the primate equivalent of the caching bird as a having a theory of mind (Povinelli and Preuss 1995) and many see this as evidence of a very high level of cognition.
Mental ‘time travel’ suggests an inclusion of past and future but, for most part, research has concentrated on memory of past events. More recently, researchers have focused precisely on whether there were instances of birds actively taking into account the future (Raby et al. 2007). In a carefully controlled experiment, the birds were trained to know that there were certain foods they would not be able to access the following morning. The experiments showed that the jays were able to store ahead of time for the eventuality that their favourite food would not be available next morning (Cheke and Clayton 2012).
In an exciting paper in 2010 (already mentioned), Bell and colleagues examined a future dimension in the context of cooperative behaviour, shining an investigative light on the social domain. As the researchers discovered, babblers engaged in advance negotiation with one another concerning sentinel duties, adjusting levels and amount of contribution to be expected and agreed upon (Bell et al. 2010). Hence they communicated about likely future investment of collaborators so that individuals could make pre-emptive adjustments to their own investment. Such information on future intentions provided by others would therefore be an important variable in the level of help an individual was willing to provide (Bell et al. 2010). The social brain hypothesis may so far have its strongest validation in the findings of this paper. It links cooperation with the need to communicate intentionally for the sake of group harmony and safety. If I interpret their paper correctly, the birds are intensely social and consider each other’s future moves. If the researchers had found a method to study future intentions in animals and others found that method scientifically acceptable, then one may expect that further studies will follow. They might even topple this assumption of human uniqueness and the concomitant view that animals only live in the present and do not remember the past and cannot think in a future dimension.
Forming abstract concepts
Research on cognition is by no means a new field. Indeed, it has about a 100-year-old history, and research on forming abstract concepts in birds is now a good five decades old. In this field of comparative psychology, it was mostly the pigeon that was tested for abstract concepts, as first reported in the 1960s by Herrnstein and Loveland (1964). Testing pigeons pecking at keys onto which photographs had been projected, showed that they could form abstract concepts of ‘oddity’ (pecking the odd picture out of a group) and ‘sphericity’ (pecking the image of any rounded shape) (O’Brian 1987). Pigeons (called feral pigeons in Australia and rock pigeons elsewhere) rank better in ‘odd-man-out’ type tasks (same/different) tasks (Castro and Wasserman 2011) than most people, they can deal with concepts and distinguish between objects and abstract concepts and conceptual categories such as ‘volume’ or ‘colour’ or ‘forest’. Pigeons can even form the abstract concept of ‘water’ on a two-dimensional screen and would peck at any image with water in it, regardless of whether the water was in a glass, a lake or a droplet on a leaf (Delius 1987). Moreover, pigeons can learn to discriminate between photographs containing, and not containing a human, or humans (Herrnstein and Loveland 1964).
Another study has demonstrated that a bird can perform better than a human on a task requiring matching of rotated objects or symbols. Juan Delius (1985) tested pigeons on a task based on a question selected from the Eyssenk IQ test for humans. Pigeons were trained to look at three keys in a row. An asymmetrical symbol was projected onto the centre key and the same symbol was projected onto one of the side keys. Onto the other side key was projected a mirror-image reversal of the symbol. The pigeon had to peck the side key matching the one in the centre in order to obtain a food reward. Once the pigeon was performing the task accurately, the symbols were rotated at different angles compared with the one on the centre key. Humans find this task more and more difficult as the rotation angles increase, just as we have difficulty in recognising a familiar face when we see it upside down, but pigeons have no difficulties in performing this task regardless of the angle of rotation (Delius 1985). The ability to make decisions whether two objects are the same or different has also been shown in pigeons (Katz and Wright 2006) and in the African Grey parrot, Alex, that replied to such questions directly using human speech (Pepperberg 1987). Pigeons have thus been tested in a very wide variety of cognitive and perceptual tasks, whether on insight or means-end tests (Cook and Fowler 2014; Schmidt and Cook 2006). They have been shown to be capable of complex learning, fine discriminations and even symbolic hue matching (Cook et al. 1995).
Young chicks have some abilities to form abstract concepts, using geometrical cues (Vallortigara et al. 1990; Tommasi and Vallortigara 2004). Recently it was found that even bees can acquire the ability to deal with conceptual relationships such as ‘above’ and ‘below’, ‘same’, ‘different’, ‘larger than’ and ‘better than’, among others (Avarguès-Weber et al. 2011; Avarguès-Weber and Giurfa 2013) and can perceive symmetry (Giurfa et al. 1996), but they may do so using quite different neural processes than birds or other vertebrates.
Hence, following several decades of research, it can now be argued with confidence that all of these elements described above – a basis in natural physics, mathematics, geometry and natural psychology (for review of these four pillars of animal cognition see Vallortigara et al. 2010) – are present in at least three groups of birds: non-songbirds, songbirds and parrots.
The concept of self-awareness, as well as the awareness of a state of others, is a matter for theory of mind and very controversial to this day. One way to test this (itself a controversial method) is to provide mirror images to test whether an animal recognises that the image in the mirror is a reflection and not a separate organism. In 2008, Prior and colleagues chose to test European black-billed magpies, hand-raised them and then exposed them to a series of tests. The crucial test was placing a red dot on the bird’s chest, in a spot where the bird could not see it directly, but only in a mirror. They found convincing evidence that the bird directed its attention to its own body and attempted to reach the spot where the red paint had been placed, rather than pecking at the reflection of the red dot in the mirror (Prior et al. 2008). So far, this has remained the only test of its kind showing self-recognition by birds in mirrors but the rigorous research protocol would suggest that, at least in this bird species, it seems likely that something akin to self-awareness is present. Other tests with birds and mirrors have failed to show that they recognise their image as self (summarised in Emery and Clayton 2004). Evidence of this is extremely scanty partly because of questions of method.
In summary, this chapter has shown without doubt, as have all previous ones, abilities that together make up a rather weighty body of evidence that birds can do many more intelligent things than had ever been assumed possible.
There is also a picture emerging that Australian native species are capable of complex cognitive behaviour and are obviously much cleverer than we ever thought. Whether Australian birds needed to be more intelligent and/or long lived to survive than in other parts of the world is, of course, not answered by these enumerations of skills and cognitive abilities. One way to interpret the social brain hypothesis is to argue that the cooperative behaviour could have been a later development in evolution because of the demands of increased needs to coordinate and communicate as part of social life. Long life and long juvenile dependency suggest an intertwining of biological needs and better overall survival and fitness.
In terms of natural selection, one may also be tempted to suggest that the time may have come for a review of the literature. Do females select the most colourful males or those with specific attributes of song or bower building or do they have other criteria for their selection? One paper recently investigated the mating success of male satin bowerbirds (Keagy et al. 2009) and asked whether cognitive ability had something to do with the female’s choices. The researchers found that males with better problem-solving abilities had more mating success than less successful males. They produced the first evidence that individuals with better problem-solving abilities are, in fact, also more attractive (Keagy et al. 2009). Of course, it does not devalue the importance of the quality of the bower, the male’s own display and the decorations used or, as we saw, also the clever illusion of space (Chapter 5). Perhaps the smarter males also showed higher aptitudes as builders and decorators. What Keagy and colleagues have shown is that the smart birds may be the winners and all the other variables may well spring from this cognitive dimension.
The findings also make evolutionary sense. If females continue to choose the smartest birds available, or attributes that go along with being smarter, there is a greater genetic likelihood that, at least in species in which females select for this trait, brains will increase in size over time. On the other hand, doubts remain on this argument. A recent paper on the spotted bowerbird found no relationship between cognitive ability and mating success (Isden et al. 2013), reiterating the research by Madden some years earlier (Madden 2003). We have thus come full circle: from description of everyday life of birds to social competence and cognitively complex abilities back to the life history of Australian birds.